The Case: A 31-year-old woman, an immigrant from Ethiopia, was admitted to hospital with a 2-week history of general weakness, cough and fever. She denied recent dental treatment or intravenous drug use and had not undergone any invasive procedures. She had a temperature of 38.8° C, with a pulse of 113 beats per minute and a blood pressure of 100/62 mm Hg. Auscultation of the lungs revealed mild basal crackles bilaterally. Her heart sounds were normal, and a systolic murmur grade 2/6 was audible at the cardiac apex. The remainder of the physical examination produced unremarkable results, and there were no signs consistent with endocarditis. An electrocardiogram showed sinus rhythm, with a P mitrale and left ventricular hypertrophy. Her erythrocyte sedimentation rate was 83 (normal range 1–25) mm/h and her hemoglobin concentration 83 (normal range 120–160) g/L with a mean corpuscular volume of 72.1 fL. Her leukocyte count was 14.88 × 109/L, and her platelet count was 267 × 109/L. The C-reactive protein level was elevated at 53.8 (normal range 0.08–3.1) mg/L. Chest radiography showed an infiltrate in the base of the right lung that was suggestive of pneumonia.
A diagnosis of infective endocarditis was considered, and intravenous ampicillin and gentamicin therapy was started. A transesophageal echocardiogram showed mild dilatation of the left ventricle and normal mitral, aortic, tricuspid and pulmonic valves with no vegetations and minimal mitral and tricuspid regurgitation. The next day Streptococcus anginosus was isolated from all blood cultures.
The patient's fever initially declined but then reappeared on the sixth day of treatment, and a repeat chest radiography showed bilateral lung infiltrates. After the patient reported diffuse low back pain, a TC 99 bone scan was performed to look for vertebral osteomyelitis, but the results were normal. No bacteremia source was identified, and no abscesses were seen on CT of the chest and abdomen.
In view of her persistent bacteremia and unexplained murmur, the patient was examined by a senior cardiologist, who noted a 3/6 continuous “machinery” murmur above the pulmonic region consistent with a patent ductus arteriosus.
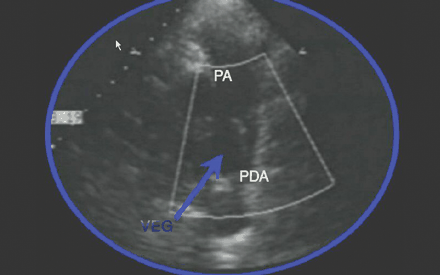
A repeat transthoracic echocardiogram, performed to look specifically for a patent ductus arteriosus, revealed a mobile mass at the left pulmonary artery that was confirmed at surgery to be 1 cm in diameter (Fig. 1). The pulmonic valve was normal (see video, available at www.cmaj.ca/cgi/content/full/174/8/1087/DC1).
Fig. 1: Transthoracic echocardiogram showing a mobile mass of vegetation (VEG) at the left pulmonary artery (PA). PDA = patent ductus arteriosus.
The final diagnosis was endocarditis on a patent ductus arteriosus, with bilateral lung infiltrates due to recurrent embolization from the vegetation at the left pulmonary artery.1 The mobile mass was removed, and the right pulmonary artery was reconnected to the left pulmonary artery, which was then reattached to the main pulmonary artery using pericardial membrane. The patient responded well and remains in good health.
The ductus arteriosus connects the descending aorta to the left pulmonary artery. Although it normally closes soon after birth, in some infants it does not close spontaneously. A patent ductus arteriosus is usually encountered in pediatric patients and accounts for 10% of cases of congenital heart disease.2 This incidence is increased among children who are born prematurely or at a high altitude, who have a history of perinatal asphyxia, or whose mother had rubella while pregnant.2 An adult with a small patent ductus arteriosus usually has no symptoms and a normal life expectancy, but one with a moderate patent ductus arteriosus may present with fatigue, dyspnea or palpitations. One-third of adults with a patent ductus arteriosus that is not surgically repaired will die of heart failure (due to volume overload caused by an increased shunting of blood from left to right), pulmonary hypertension or endocarditis by the age of 40; two-thirds will die by the age of 60.2
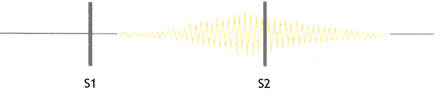
The typical continuous murmur in a patent ductus arteriosus is caused by blood flow from the high pressure descending thoracic aorta to the low pressure pulmonary artery (Gibson's murmur or machinery murmur) (a recording is available at www.cardiologysite.com/auscultation/ppchtml/pda.html). Classically, the first heart sound is normal, and the continuous “machinery” murmur is audible in the second left intercostal space beginning shortly after the first heart sound. This rough-sounding murmur peaks in intensity at or immediately after the second heart sound and declines in intensity during diastole. The second heart sound may be masked by the continuous murmur. If pulmonary hypertension develops, the continuous murmur decreases in duration and intensity and eventually disappears, and a diastolic decrescendo murmur may appear as a result of concomitant pulmonary regurgitation2,3 (Fig. 2). Echocardiographic detection of vegetation within the pulmonary artery is extremely rare and challenging.
Fig. 2: Schematic presentation of auscultatory findings in a patent ductus arteriosus. S1 and S2 denote the first and second heart sounds. The continuous murmur starts after S1, peaks near S2 and declines during diastole. The second heart sound may be masked by the murmur.
Lung involvement with persistent bacteremia and murmur suggests patent ductus arteriosus-related endocarditis. A small patent ductus arteriosus may not be detected during routine medical care. This case provides a reminder that good clinical skills, including proficient cardiac auscultation, is often the most reliable diagnostic tool.
Footnotes
-
Competing interests: None declared.