Abstract
Background: There have been postmarketing reports of adverse cardiovascular events associated with the use of varenicline, a widely used smoking cessation drug. We conducted a systematic review and meta-analysis of randomized controlled trials to ascertain the serious adverse cardiovascular effects of varenicline compared with placebo among tobacco users.
Methods: We searched MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, websites of regulatory authorities and registries of clinical trials, with no date or language restrictions, through September 2010 (updated March 2011) for published and unpublished studies. We selected double-blind randomized controlled trials of at least one week’s duration involving smokers or people who used smokeless tobacco that reported on cardiovascular events (ischemia, arrhythmia, congestive heart failure, sudden death or cardiovascular-related death) as serious adverse events asociated with the use of varenicline.
Results: We analyzed data from 14 double-blind randomized controlled trials involving 8216 participants. The trials ranged in duration from 7 to 52 weeks. Varenicline was associated with a significantly increased risk of serious adverse cardiovascular events compared with placebo (1.06% [52/4908] in varenicline group v. 0.82% [27/3308] in placebo group; Peto odds ratio [OR] 1.72, 95% confidence interval [CI] 1.09–2.71; I2 = 0%). The results of various sensitivity analyses were consistent with those of the main analysis, and a funnel plot showed no publication bias. There were too few deaths to allow meaningful comparisons of mortality.
Interpretation: Our meta-analysis raises safety concerns about the potential for an increased risk of serious adverse cardiovascular events associated with the use of varenicline among tobacco users.
See related commentary by Hays on page 1346 and at www.cmaj.ca/lookup/doi/10.1503/cmaj.110804 and related letters on page 1404.
Varenicline is one of the most widely used drugs for smoking cessation. It is a partial agonist at the α4–β2 nicotinic acetylcholine receptors and a full agonist at the α7 nicotinic acetylcholine receptor.1,2 The drug modulates parasympathetic output from the brainstem to the heart because of activities of the α7 receptor.3 Acute nicotine administration can induce thrombosis.4 Possible mechanisms by which varenicline may be associated with cardiovascular disease might include the action of varenicline at the α7 receptor in the brainstem or, similar to nicotine, a prothrombotic effect.2–4
At the time of its priority safety review of varenicline in 2006, the US Food and Drug Administration (FDA) noted that “[t]he serious adverse event data suggest that varenicline may possibly increase the risk of cardiac events, both ischemic and arrhythmic, particularly over longer treatment period.”5 Subsequently, the product label was updated: “Post marketing reports of myocardial infarction and cerebrovascular accidents including ischemic and hemorrhagic events have been reported in patients taking Chantix.”6 There are published reports of cardiac arrest associated with varenicline.7
Cardiovascular disease is an important cause of morbidity and mortality among tobacco users. The long-term cardiovascular benefits of smoking cessation are well established.8 Although one statistically underpowered trial reported a trend toward excess cardiovascular events associated with the use of varenicline,9 a systematic review of information on the cardiovascular effects of varenicline is unavailable to clinicians.
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to ascertain the serious adverse cardiovascular effects of varenicline compared with placebo among tobacco users.
Methods
Literature search
We initially searched MEDLINE and EMBASE in September 2010 using free text and indexing terms for “varenicline” and “clinical trials.” At the same time, we identified unpublished studies from the websites of regulatory authorities (the FDA and the European Medicines Agency), as well as results of clinical trials of varenicline included in the ClinicalTrials.gov Results Database (www.clinicaltrials.gov/ct2/info/results) and the industry-sponsored Clinical Study Results Database (www.clinicalstudyresults.org/home). In March 2011, we conducted an updated search using an optimized filter for MEDLINE and an RCT filter for EMBASE; we also searched the Cochrane Central Register of Controlled Trials. We evaluated the bibliographies of included trials and recent systematic reviews, Cochrane reviews1 and meta-analyses for relevant RCTs. We did not have any language restrictions. Details of our search strategy appear in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110218/-/DC1).
We selected double-blind RCTs with at least one week of follow-up that evaluated varenicline as the intervention drug versus a placebo among tobacco users and that reported on cardiovascular events (including no events). We excluded RCTs involving non-tobacco users and observational studies.
We chose the minimum follow-up period of one week to ascertain the early cardiovascular effects of varenicline, because the half-life of the drug is about 24 hours and at least five half-lives are needed to reach a steady state.10 Our primary analysis focused on double-blind placebo-controlled trials, because blinding is critical to the adequate ascertainment of serious adverse events and because placebo-controlled trials provide unconfounded estimates of treatment effect. In addition, nicotine replacement therapy may be associated with cardiovascular risk.11 Open-label trials and trials of varenicline and active comparators were systematically identified and evaluated in a sensitivity analysis.
Outcome measures
The primary outcome was any ischemic or arrhythmic adverse cardiovascular event (myocardial infarction, unstable angina, coronary revascularization, coronary artery disease, arrhythmias, transient ischemic attacks, stroke, sudden death or cardiovascular-related death, or congestive heart failure) reported by the investigators during the double-blind period of the trial. We evaluated all-cause mortality as a secondary outcome.
Data abstraction
We scanned all titles and abstracts of studies identified through our searches and excluded articles that clearly did not meet the selection criteria. We evaluated full-text versions of the remaining articles for their eligibility to be included in the review. We evaluated trials listing adverse events and recorded numerical data on adverse cardiovascular events and specific descriptions of cardiovascular events in the studies up to the completion of the specified follow-up period. We collected information on the study design, the phase of the trial, the location of the study, any exclusions from enrolment of patients with cardiac conditions and significant cardiac risk factors, the dose of varenicline, the patients’ mean age, the proportion of patients who were men, the ethnicity, the duration of tobacco use and relevant outcomes. To avoid potential duplication, we reconciled studies published in journals with trial reports from the manufacturer and regulatory authorities.
Assessment of risk of bias
We evaluated the studies for adequacy of sequence generation, allocation concealment, blinding of participants and personnel, reporting of withdrawals and loss to follow-up, and reporting of adverse outcomes.12 Two of us (S.S. and J.G.S. or Y.K.L.) were independently involved in all stages of study selection, data extraction and quality assessment. All discrepancies were resolved after rechecking the source papers and further discussion among the reviewers, with arbitration by a third reviewer (C.D.F) and full consensus before inclusion.
Data synthesis
We used Review Manager (RevMan version 5.025; Nordic Cochrane Center, Copenhagen, Denmark) to conduct the meta-analysis. The unit of analysis was individuals with adverse cardiovascular events. Analysis was by intention to treat and included all participants, including dropouts, to minimize bias due to differences in dropout numbers between groups. We used the Peto method to calculate odds ratios (ORs) and 95% confidence intervals (CIs) because this method provides the best confidence interval coverage and is more powerful and relatively less biased than the random-effects analysis when dealing with low event rates.13 Statistical heterogeneity was assessed using the I2 statistic, with values of 50% or more indicating a substantial level of heterogeneity.14 Statistical significance was set at two-sided α of 0.05. In trials that had more than two intervention groups, we preserved randomization but collapsed the multiple intervention arms (e.g., varenicline 0.25 mg twice daily and 0.5 mg twice daily) into single treatment arms.12
To account for potential imbalance in trial size and in the number of studies with zero events, prespecified sensitivity analyses were conducted using the fixed Mantel–Haenszel test.14,15 Prespecified sensitivity analyses were also conducted to determine the influence on effect size of the choice of comparators (placebo v. active controls), and of the role of individual trials by excluding the most influential trial.9 To maintain similarity of investigator-reported definitions of cardiovascular events across the trials, we conducted sensitivity analyses to determine the robustness of effect size when unadjudicated cardiovascular events were added from the trial that reported adjudicated events.9 We evaluated the effects of limiting our analysis to trials that used a similar dose of varenicline (1 mg twice daily) or to trials that reported only on specific cardiovascular outcomes of myocardial infarction, stroke and cardiovascular-related death. Publication bias was estimated via examination of asymmetry in a funnel plot.
The protocol is available on request from the corresponding author.
Results
Study characteristics
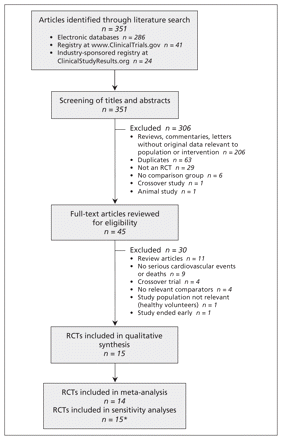
The selection of studies included in our review is summarized in Figure 1. Fourteen double-blind placebo-controlled trials were included in the meta-analysis.9,16–28 An additional open-label trial of varenicline versus nicotine replacement therapy was included in the sensitivity analysis.29
Selection of double-blind placebo-controlled randomized controlled trials (RCTs) for inclusion in the systematic review and meta-analysis of the risk of serious adverse cardiovascular events associated with varenicline use. *An additional open-label trial of varenicline versus nicotine replacement therapy was included in the sensitivity analysis.
Characteristics of the trials are summarized in Table 1 and Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110218/-/DC1). The 14 double-blind placebo-controlled trials enrolled a total of 8216 patients (4908 in the varenicline arms, 3308 in the placebo arms). The sample sizes ranged from 250 to 1210. The duration of treatment ranged from 7 weeks to 52 weeks, and the total duration of study, including treatment and follow-up, ranged from 24 to 52 weeks. All but one of the trials enrolled smokers; the remaining trial enrolled people who used smokeless tobacco.18 The primary outcome was the continuous abstinence rate in 12 trials,9,16–25,27 the long-term quit rate in 1 trial26 and long-term safety in 1 trial.28 All but one of the trials excluded patients with a history of cardiovascular disease; the remaining trial included participants with stable cardiovascular disease but excluded those with unstable cardiovascular disease.9 Two of the 14 RCTs also had a bupropion comparator group.19,20 In most of the trials, the dose of vareniciline was 1 mg twice daily. Three trials reported on lower doses of varenicline.21,23,24
Characteristics of randomized controlled trials of varenicline included in the analysis of serious adverse cardiovascular events*
Details of the risk-of-bias assessment appear in Table 2 and Appendix 3 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110218/-/DC1). Nine RCTs were judged to be at low risk of bias (adequate sequence generation, allocation concealement and double blinding, and clear reporting of withdrawal rates);9,18–23,26,27 the remaining five RCTs were at unclear risk of bias owing to incomplete reporting of randomization.16,17,24,25,28 The open-label trial had an unclear risk of bias.29 Loss to follow-up ranged from as low as none (in a treatment arm24 and a placebo arm27) to as high as 28.6% (in a placebo arm24); loss to follow-up was higher in the placebo arm in most of the trials except three, in which it was higher in the varenicline arm.9,18,27
Risk-of-bias assessment of randomized controlled trials (RCTs) of varenicline included in the analysis of serious adverse cardiovascular events*
Thirteen trials did not use an objective definition of cardiovascular events and evaluated such events as serious if they resulted in hospital admission, disability or death.16–28 Although the role of adjudication of major clinical events remains unclear,30 one trial adjudicated cardiovascular events and reported on investigator-reported cardiovascular events that did not reach the threshold for adjudication.9 All of the included trials reported on mortality.9,16–28 Data on adverse cardiovascular events and mortality are shown in Appendix 4 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110218/-/DC1).
Risk of serious adverse cardiovascular events and death
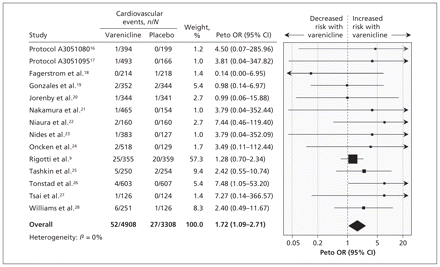
The meta-analysis showed a significantly increased risk of serious adverse cardiovascular events associated with varenicline compared with placebo (Peto OR 1.72, 95% CI 1.09–2.71; I2 = 0%) (Figure 2).9,16–28
Meta-analysis of double-blind placebo-controlled randomized trials of the risk of serious adverse cardiovascular events associated with the use of varenicline. An odds ratio (OR) greater than 1.0 indicates an increased risk of a serious adverse cardiovascular event. CI = confidence interval.
Only five trials reported deaths (7/4908 in the varenicline group v. 7/3308 in the placebo group.9,19,21,25,26 This precluded any pooling of such sparse data in a meta-analysis.
Sensitivity analyses
Sensitivity analyses9,16–28 using the reciprocal of the treatment arm with a continuity correction (fixed Mantel–Haenszel OR 1.67, 95% CI 1.06–2.64) or without a continuity correction (fixed Mantel–Haenszel OR 1.77, 95% CI 1.09–2.88) showed results similar to those of the primary analysis (Table 3). The sensitivity analysis in which we included data for active comparators (nicotine replacement therapy in the open-label trial29 and bupropion in two placebo-controlled trials19,20) showed results similar to those of the the primary analysis (Peto OR 1.67, 95% CI 1.07–2.62).9,16–29 When we excluded the most influential trial (which involved participants with stable cardiovascular disease),9 the results were also similar to those of the primary analysis (Peto OR 2.54, 95% CI 1.26–5.12).16–28 The same was true when we included investigator-reported cardiovascular events from a trial that did not meet the threshold for adjudication9 (Peto OR 1.91, 95% CI 1.25–2.94)9,16–28 (Table 3).
Sensitivity analyses for the outcome of serious adverse cardiovascular events*
In the sensitivity analysis in which we excluded data for varenicline doses less than 1 mg twice daily, the results were again similar to those of the primary analysis (Peto OR 1.76, 95% CI 1.11–2.77).9,16–20,22,25–28 The data from the treatment arms that used lower doses (0.5 mg twice daily, 0.3 mg/d and 0.25 mg twice daily) were too sparse to be pooled in a meta-analysis.
Only five trials reported on specific outcomes of myocardial infarction, stroke and cardiovascular-related death;9,19,22,25,28 the sensitivity analysis of these limited data yielded a Peto OR of 1.80 (95% CI 0.83–3.91) which did not reach statistical significance.
There was no evidence of publication bias for the primary outcome (see the funnel plot in Appendix 5, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110218/-/DC1).
Interpretation
The use of varenicline among tobacco users was associated with a 72% increased risk of serious adverse cardiovascular events. The robustness of the effect size to alternative statistical approaches or comparators in various sensitivity analyses suggests that this safety signal deserves further investigation. Although one can never entirely rule out chance occurrence, there are potential alternative explanations for these findings. One possibility is that the participants in the placebo arms experienced a lower rate of serious adverse cardiovascular events because of failure of randomization. A systematic failure of randomization is unlikely, because the baseline characteristics of the participants were well balanced between groups. However, despite achieving more than twofold higher rates of abstinence in the trials, which should potentially induce a cardiovascular benefit, the participants taking varenicline experienced an increased risk of serious adverse cardiovascular events.
Our finding needs to be interpreted in the context of other studies and the overall benefit–risk profile of varenicline. An earlier pooled analysis of data from clinical trials identified 22 serious ischemic and arrhythmic cardiac adverse events among 3940 patients allocated to receive varenicline compared with 4 such events among 1209 patients allocated to receive placebo (2.32 per 100 patient exposure-years for varenicline v. 1.63 per 100 patient exposure-years for placebo after accounting for differences in exposure).5 Our meta-analysis extends the findings of this analysis: including the data from those studies plus the data from several other published studies, we found three times the number of serious adverse cardiovascular events among participants in the varenicline group (61/4908 for varenicline v. 29/3308 for placebo).
Varenicline increases the chances of a successful quit attempt by twofold compared with unassisted smoking cessation.1 However, at the population level, most smokers quit unassisted.31 The number needed to treat with varenicline for one additional person to successfully quit smoking is estimated to be 10 (95% CI 8–13).1 Assuming a baseline risk of serious adverse cardiovascular events of 5.57% per year (among smokers with stable cardiovascular disease),9 the number needed to harm (the number needed to cause one additional serious cardiovascular event) with varenicline is estimated to be 28 (95% CI 13 to 213) per year. The risk of additional serious adverse events associated with varenicline use includes the potential for serious neuropsychiatric symptoms such as depressed mood, agitation and suicidal thoughts.32 These additional risks associated with varenicline have resulted in a boxed warning (the highest level of FDA warning) in the medication guide and a Risk Evaluation and Mitigation Strategy for varenicline in the United States.32 All smoking cessation therapies are approved for short-term use. Long-term efficacy and safety data are lacking for all currently approved therapies, including bupropion.
Limitations
The limitations of our meta-analysis stem mainly from the quality of reported summary data. The trials enrolled different populations, evaluated different doses of varenicline and had different lengths of follow-up and proportions lost to follow-up. Our estimates are imprecise owing to the low event rates. None of the trials was adequately powered to detect individual differences in cardiovascular events. Although the included trials were double blinded, differences in ascertainment mediated by the cardiac symptoms of nicotine withdrawal is possible. In the absence of source data, we could not assess for potential blinding failure, blinding biases or differences in ascertainment, or determine whether these events were immediate or delayed.33 The cardiovascular events were not prespecified. Thus, we could not determine whether the diagnoses were clinical diagnoses or confirmed by established diagnostic criteria. Finally, the applicability of our findings to smokers with unstable cardiovascular disease remains uncertain because these people were excluded from the trials.
Conclusion
Our meta-analysis raises safety concerns about the potential for an increased risk of serious adverse cardiovascular events associated with the use of varenicline among tobacco users. Despite the limitations of our analysis, our findings have potential regulatory and clinical implications. Drugs that receive priority review have limited safety data at the time of approval.34 The initial safety signal regarding cardiovascular events in people using varenicline was not followed up by an adequately powered safety trial. Until such trials are conducted, clinicians should carefully balance the risk of serious cardiovascular events and serious neuropsychiatric adverse events associated with varenicline use against the known benefits of the drug on smoking cessation.
Addendum
In June 2011, the FDA announced the addition of a warning to the product label of Chantix (varenicline) about the small, increased risk of certain adverse cardiovascular events associated with the use of varenicline among smokers with cardiovascular disease.35
Footnotes
-
Competing interests: Curt Furberg was paid by plaintiffs for expert testimony on Pfizer’s COX-2 inhibitors. No competing interests declared by the other authors.
-
This article has been peer reviewed.
-
Contributors: Sonal Singh, John Spangler and Yoon Loke were responsible for the study concept and design and for the acquisition of data. Sonal Singh and Yoon Loke performed the statistical analysis. Curt Furberg supervised the study. All of the authors analyzed and interpreted the data, drafted the manuscript and critically revised the manuscript for important intellectual content. Sonal Singh had full access to all of the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis and acts as guarantor of the paper.
-
Funding: Sonal Singh is supported by a grant from the National Center for Research Resources (NCRR), a component of the US National Institutes of Health (NIH), and the NIH Roadmap for Medical Research (grant no. 1KL2RR025006-03). The design and conduct of the study; the collection, management, analysis and interpretation of the data; and the preparation, review and approval of the manuscript were independent of any sources of funding. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or the NIH.