- © 2005 CMA Media Inc. or its licensors
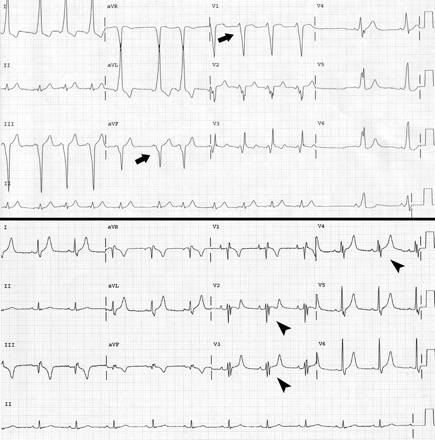
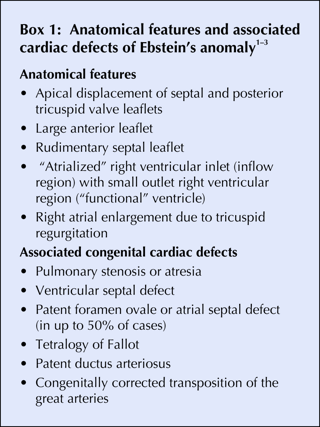
Fig. 1: Top: Electrocardiogram from patient with Ebstein's anomaly of the tricuspid valve, showing shortened PR interval, prolonged QRS interval and delta waves (arrows). Bottom: Electrocardiogram after radiofrequency ablation, showing prolonged PR interval and odd “second” QRS complex in leads III, aVF and V2–V4 (arrowheads), a consequence of abnormal impulse conduction in the “atrialized” right ventricle.
CASE: A 28-year-old previously healthy man presented with a 6-week history of palpitations. The symptoms occurred during rest, 2–3 times per week, lasted up to 30 minutes at a time and were associated with dyspnea. Except for a grade 2/6 holosystolic tricuspid regurgitation murmur (best heard at the left sternal border with inspiratory accentuation), physical examination yielded unremarkable findings. An electrocardiogram (ECG) revealed normal sinus rhythm and a Wolff– Parkinson– White pre-excitation pattern (Fig. 1: Top), produced by a right-sided accessory pathway. Transthoracic echocardiography demonstrated the presence of Ebstein's anomaly of the tricuspid valve, with apical displacement of the valve and formation of an “atrialized” right ventricle (a functional unit between the right atrium and the inlet [inflow] portion of the right ventricle) (Fig. 2). The anterior tricuspid valve leaflet was elongated (Fig. 2C, arrow), whereas the septal leaflet was rudimentary (Fig. 2C, arrowhead). Contrast echocardiography using saline revealed a patent foramen ovale with right-to-left shunting and bubbles in the left atrium (Fig. 2D).
Fig. 2: A: Echocardiogram showing Ebstein's anomaly (apical displacement) of the tricuspid valve (arrow) and formation of an “atrialized” right ventricle (ARV). B: Anatomical sketch of echocardiogram in A. C: Echocardiogram showing elongated anterior tricuspid valve leaflet (arrow) and rudimentary septal leaflet (arrowhead). D: Contrast echocardiogram using saline, showing patent foramen ovale with right-to-left shunting and bubbles in the left ventricle (arrow). RA = right atrium, LA = left atrium.
The patient underwent an electrophysiologic study with mapping of the accessory pathway, followed by radiofrequency ablation (interruption of the pathway using the heat generated by electromagnetic waves at the tip of an ablation catheter). His post-ablation ECG showed a prolonged PR interval and an odd “second” QRS complex in leads III, aVF and V2–V4 (Fig. 1Bottom), a consequence of abnormal impulse conduction in the “atrialized” right ventricle. The patient reported no recurrence of palpitations at follow-up 6 months after the ablation.
The tricuspid valve anomaly described by Ebstein in 1864 consists of apical displacement of the septal and posterior tricuspid leaflets, which results in an enlarged right atrium functionally integrated with the inlet region of the right ventricle (“atrialized” right ventricle). The outlet and trabecular portions of the right ventricle constitute an often hypoplastic, “functional” ventricle (Box 1). Ebstein anomaly occurs in 5 per 100 000 live births, accounting for 0.5% of all cases of congenital heart disease.1 Risk factors believed to be associated with the condition are a family history of Ebstein's anomaly or other congenital heart disease, northern European ancestry and maternal exposure to benzodiazepines or lithium.1,2,3 More than 30% of patients with Ebstein's anomaly have associated cardiac defects1 (Box 1).
The clinical manifestations of Ebstein's anomaly (Box 2) depend on the degree of tricuspid valve malformation and consequent regurgitation, and any associated cardiac defects.2,3 Many patients first experience symptoms as adults, but the onset can occur after birth or in infancy or childhood. In newborns, the anomaly often presents as cyanosis and, in the absence of surgical repair, is associated with a 20% mortality in the first year of life. In infants, it may present as congestive heart failure and in children as an incidental murmur. In adults, the anomaly commonly presents with arrhythmias. Factors associated with a worse outcome are young age at diagnosis, male sex, cardiothoracic ratio of more than 0.65 and the presence of cyanosis.2
The treatment of Ebstein's anomaly has to be tailored to the individual patient. Patients with heart failure and little impairment in functional capacity can be managed medically. Atrial arrhythmias without evidence of pre-excitation can be treated pharmacologically, whereas percutaneous radiofrequency ablation is indicated in the presence of an accessory pathway. In general, surgical intervention with tricuspid valve repair or replacement is restricted to patients with severe heart failure, cyanosis, intractable arrhythmias or paradoxical embolization (passage of thrombi from the venous circulation into the arterial circulation through a right-to-left shunt at the atrial level). Patients with Ebstein's anomaly should be assessed regularly for signs of deterioration in functional capacity, increasing cyanosis or presence of arrhythmia. Prophylaxis against infective endocarditis is warranted in all cases.
Electrocardiographic evidence of pre-excitation (delta wave on upstroke of QRS complex) is found in up to 0.25% of the general population. The incidental finding of pre-excitation in an otherwise asymptomatic patient with normal cardiac findings on physical examination does not warrant further investigation. The patient should be advised to report any symptoms suggestive of tachyarrhythmia. Patients with Wolff–Parkinson–White syndrome (pre-excitation and symptomatic tachyarrhythmia) should be referred to a specialist for electrophysiologic evaluation. The presence of pre-excitation and a tricuspid regurgitation murmur, as in the case we have described, should raise the suspicion of Ebstein's anomaly.