Abstract
Background: The gut microbiota is essential to human health throughout life, yet the acquisition and development of this microbial community during infancy remains poorly understood. Meanwhile, there is increasing concern over rising rates of cesarean delivery and insufficient exclusive breastfeeding of infants in developed countries. In this article, we characterize the gut microbiota of healthy Canadian infants and describe the influence of cesarean delivery and formula feeding.
Methods: We included a subset of 24 term infants from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort. Mode of delivery was obtained from medical records, and mothers were asked to report on infant diet and medication use. Fecal samples were collected at 4 months of age, and we characterized the microbiota composition using high-throughput DNA sequencing.
Results: We observed high variability in the profiles of fecal microbiota among the infants. The profiles were generally dominated by Actinobacteria (mainly the genus Bifidobacterium) and Firmicutes (with diverse representation from numerous genera). Compared with breastfed infants, formula-fed infants had increased richness of species, with overrepresentation of Clostridium difficile. Escherichia–Shigella and Bacteroides species were underrepresented in infants born by cesarean delivery. Infants born by elective cesarean delivery had particularly low bacterial richness and diversity.
Interpretation: These findings advance our understanding of the gut microbiota in healthy infants. They also provide new evidence for the effects of delivery mode and infant diet as determinants of this essential microbial community in early life.
See related commentary by Song and colleagues on page 373 and at www.cmaj.ca/lookup/doi/10.1503/cmaj.130147
The human body harbours trillions of microbes, known collectively as the “human microbiome.” By far the highest density of commensal bacteria is found in the digestive tract, where resident microbes outnumber host cells by at least 10 to 1. Gut bacteria play a fundamental role in human health by promoting intestinal homeostasis, stimulating development of the immune system, providing protection against pathogens, and contributing to the processing of nutrients and harvesting of energy.1,2 The disruption of the gut microbiota has been linked to an increasing number of diseases, including inflammatory bowel disease, necrotizing enterocolitis, diabetes, obesity, cancer, allergies and asthma.1 Despite this evidence and a growing appreciation for the integral role of the gut microbiota in lifelong health, relatively little is known about the acquisition and development of this complex microbial community during infancy.3
Two of the best-studied determinants of the gut microbiota during infancy are mode of delivery and exposure to breast milk.4,5 Cesarean delivery perturbs normal colonization of the infant gut by preventing exposure to maternal microbes, whereas breastfeeding promotes a “healthy” gut microbiota by providing selective metabolic substrates for beneficial bacteria.3,5 Despite recommendations from the World Health Organization,6 the rate of cesarean delivery has continued to rise in developed countries and rates of breastfeeding decrease substantially within the first few months of life.7,8 In Canada, more than 1 in 4 newborns are born by cesarean delivery, and less than 15% of infants are exclusively breastfed for the recommended duration of 6 months.9,10 In some parts of the world, elective cesarean deliveries are performed by maternal request, often because of apprehension about pain during childbirth, and sometimes for patient–physician convenience.11
The potential long-term consequences of decisions regarding mode of delivery and infant diet are not to be underestimated. Infants born by cesarean delivery are at increased risk of asthma, obesity and type 1 diabetes,12 whereas breastfeeding is variably protective against these and other disorders.13 These long-term health consequences may be partially attributable to disruption of the gut microbiota.12,14
Historically, the gut microbiota has been studied with the use of culture-based methodologies to examine individual organisms. However, up to 80% of intestinal microbes cannot be grown in culture.3,15 New technology using culture-independent DNA sequencing enables comprehensive detection of intestinal microbes and permits simultaneous characterization of entire microbial communities. Multinational consortia have been established to characterize the “normal” adult microbiome using these exciting new methods;16 however, these methods have been underused in infant studies. Because early colonization may have long-lasting effects on health, infant studies are vital.3,4 Among the few studies of infant gut microbiota using DNA sequencing, most were conducted in restricted populations, such as infants delivered vaginally,17 infants born by cesarean delivery who were formula-fed18 or preterm infants with necrotizing enterocolitis.19
Thus, the gut microbiota is essential to human health, yet the acquisition and development of this microbial community during infancy remains poorly understood.3 In the current study, we address this gap in knowledge using new sequencing technology and detailed exposure assessments20 of healthy Canadian infants selected from a national birth cohort to provide representative, comprehensive profiles of gut microbiota according to mode of delivery and infant diet.
Methods
Study design
This descriptive study included 24 term (37–41 weeks’ gestation) infants whose mothers were recruited at the Winnipeg, Manitoba, site of the Canadian Healthy Infant Longitudinal Development (CHILD) population-based birth cohort (www.canadianchildstudy.ca). Pregnant women were enrolled between November 2008 and August 2009. We selected the first 24 infants for whom fecal samples were available for analysis. Mode of delivery, timing of membrane rupture, use of maternal antibiotics and group B streptococcus status were obtained from hospital records. Stool samples were collected from the infants at age 3–4 months. Mothers were asked to complete a questionnaire for information about infant diet (categorized as exclusive, partial or no breastfeeding) and medication use. Informed consent was obtained from parents at enrolment. This study was approved by the University of Manitoba Health Research Ethics Board.
Analysis of fecal samples
Complete protocols are included in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.121189/-/DC1). In brief, bacterial DNA was extracted from stool samples using the FastPrep DNA for Soil Kit (MP Biomedicals, Solon, Ohio), followed by high-throughput signature gene sequencing to identify individual organisms. The signature gene used was 16S rRNA, which is ubiquitous among bacteria and contains variable regions that can be used to identify individual species. The variable regions V5, V6 and V7 were amplified, sequenced and classified according to SILVA taxonomy (www.arb-silva.de), as described previously.21 We used quantitative polymerase chain reaction as described by Penders and colleagues5 for targeted analysis of Clostridium difficile because of the organism’s association with atopic allergic disease in a previous study.25
Statistical analysis
In this descriptive study, we report fecal microbiome biodiversity and relative abundance of bacterial taxa according to mode of delivery and infant diet. Biodiversity measures — the Chao1 score and the Shannon diversity index — were calculated using the open-source software package QIIME (Quantitative Insights into Microbial Ecology, http://qiime.org/) with rarefied data (10 000 sequences per sample). The Chao1 score estimates the number of different species present and the Shannon diversity index evaluates both the number of species and the evenness of their distribution. Taking advantage of our sample size (larger than many in sequencing-based studies of infant gut microbiota), we performed statistical comparisons using analysis of variance, the t test, Spearman rank-order correlation or the Cochrane–Armitage χ2 test for trend, as indicated. We assessed differential abundance of bacterial taxa at the family and genus levels using Metastats (http://metastats.cbcb.umd.edu). Metastats is a statistically rigorous method designed specifically to compare microbial communities on the basis of 16S rRNA abundance data. To account for multiple testing, Metastats computes q values, which may be interpreted as p values adjusted for multiple comparisons.
Results
Study population
Fecal samples were collected from 24 healthy term infants (mean age ± standard deviation 17.4 ± 3.2 weeks). The study population comprised equal numbers of boys and girls; 6 infants (25%) were born by cesarean delivery (Table 1). At the time of sampling, 10 (42%) of the infants were exclusively breastfed, 5 (21%) were partially breastfed (supplemented with formula), and 9 (38%) were not breastfed. Exclusive breastfeeding was more common among infants born vaginally than among those born by cesarean delivery (44% v. 33%). Of the mothers, 11 (46%) received antibiotics during pregnancy or at delivery, including 5 (28%) of the mothers who had a vaginal delivery and all of those who had cesarean delivery. Three infants received antibiotics directly.
Characteristics of term infants in study population
Profiles of gut microbiota
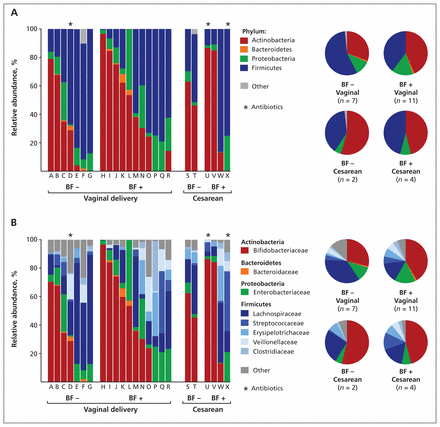
The relative abundance of dominant bacterial phyla, families and genera for each infant is shown in Figure 1, with median values presented in Table 2. Fecal microbiota profiles were generally dominated by the phylum Actinobacteria (median 36.4%, with representation mainly by the genus Bifidobacterium) and Firmicutes (median 43.8%, with diverse representation from numerous genera). A strong negative correlation was observed between these dominant phyla (r = −0.92, p < 0.001). Bacteria in the phylum Proteobacteria were less abundant (median 7.4%) but were present in all of the infants. In contrast, organisms in the phylum Bacteroidetes were detected in less than half (37.5%) of the study population; this phylum was absent from all infants born by cesarean delivery, regardless of breastfeeding status (Figure 1).
Composition of fecal microbiota in 24 healthy infants (mean age 4 mo), at the phylum (A) and family (B) level, by mode of delivery and diet. Each column represents 1 infant, as described in Table 1. BF − = no breastfeeding, BF + = exclusive or partial breastfeeding from birth until fecal sampling.
Relative abundance and frequency of dominant phyla, families and genera in fecal samples obtained at 4 months from 24 healthy Canadian infants
Profiles of gut microbiota by mode of delivery and diet
Despite high variability between infants, we were able to detect significant effects of mode of delivery and diet on the relative abundances of several bacterial taxa (Table 3). Compared with infants who were delivered vaginally, those born by cesarean delivery had bacterial communities with significantly lower abundances of Escherichia–Shigella (p < 0.001, q = 0.001) and an absence of Bacteroides (p = 0.02, q = 0.03).
Relative abundance of dominant phyla, families and genera in fecal samples, by mode of delivery and infant diet*
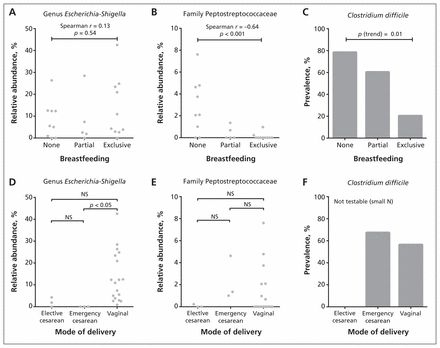
Compared with infants who were breastfed, those who were not breastfed had bacterial communities with significantly higher abundances of the family Peptostreptococcaceae (p = 0.002, q = 0.01) and the family Verrucomicrobiaceae (genus Akkermansia) (p = 0.001, q = 0.01). The prevalence of C. difficile (a member of the Peptostreptococcaceae family, not the Clostridiaceae family) was significantly lower among exclusively breastfed infants than among infants receiving formula (20% v. 71%, p = 0.01); the prevalence did not differ by mode of delivery.
We did not conduct stratified comparisons of delivery mode by infant diet because of small group sizes. Because few infants received antibiotics, we were unable to test for direct effects of antibiotics. We detected no significant differences according to intrapartum exposure to antibiotics, although the genus Blautia tended to be overrepresented among exposed infants (data not shown).
Based on the results from dichotomous comparisons, we conducted follow-up analyses for 3 of the most differentially abundant taxa: Peptostreptococcaceae, Escherichia–Shigella and C. difficile (Figure 2). Exposure groups were subcategorized, with diet classified as partial, exclusive or no breastfeeding, and mode of delivery classified as vaginal, emergency cesarean or elective cesarean. Trend testing indicated that exclusivity of breastfeeding was negatively correlated with the relative abundance of Peptostreptococcaceae (Spearman r = −0.64, p < 0.001) and prevalence of C. difficile (p for trend = 0.01). Three-group comparisons revealed that the Escherichia–Shigella lineage was underrepresented in the elective and emergency cesarean groups, attaining statistical significance in the latter compared with vaginal delivery (p < 0.05). Consistent with the dichotomous comparisons, there was no diet-associated trend in the relative abundance of Escherichia–Shigella and no differences associated with mode of delivery for Peptostreptococcaceae.
Association of infant gut microbiota with mode of delivery and diet. We performed statistical comparisons using Spearman rank correlation (A and B), nonparametric analysis of variance followed by the Dunn post hoc test for multiple comparisons (D and E) and the Cochrane–Armitage χ2 test for trend (C). NS = not significant.
Richness and diversity
Overall, the mean rarefied Chao1 score for species richness was 12.0 (range 3–22). The mean Shannon diversity index was 1.38, ranging from 0.17 to 2.36 (Table 4). Formula-fed infants had increased richness compared with breastfed infants (p = 0.006) and tended to have higher diversity. When classified by mode of delivery, infants born by elective cesarean delivery had the lowest richness and diversity.
Richness and diversity of fecal microbiota in infants, by early-life exposures
Certain taxa were correlated with bacterial richness and diversity. The relative abundance of Peptostreptococcaceae was positively correlated with both richness (r = 0.52, p = 0.01) and diversity (r = 0.50, p = 0.01). The Escherichia–Shigella abundance was negatively correlated with richness (r = −0.41, p < 0.05), and the genus Bifidobacterium was negatively correlated with diversity (r = −0.57, p = 0.004). These and other correlations are shown in Table 5.
Correlation of richness and diversity of fecal microbiota with relative abundance of dominant phyla, families and genera
Interpretation
Using new high-throughput gene sequencing technology, we have characterized the gut microbiota of healthy term infants and reported differences according to mode of delivery and infant diet. The infants included in our study were part of the CHILD national birth cohort. With its population-based sample and detailed exposure assessments, this cohort is an ideal platform from which to study the early-life development of the gut microbiota. Our study addresses an important knowledge gap, since the infant gut microbiota has rarely been characterized with sequencing methods that provide sufficient coverage of the entire bacterial community.3 Our findings are particularly timely given the recent affirmation of the gut microbiota as a “super organ” with diverse roles in health and disease,1 and the increasing concern over rising rates of cesarean delivery and insufficient exclusive breastfeeding in Canada.9,10
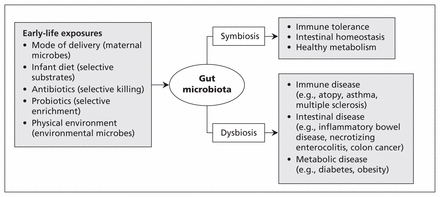
Influenced by a variety of early-life exposures, the infant gut microbiota plays a crucial role in lifelong health (Figure 3). Our current findings illustrate how this essential microbial community is modified by mode of delivery and infant diet.
Exposures in early life, infant gut microbiota and future health. Colonization of the infant intestine is influenced by various factors.4,5,14,22,23 The resulting gut microbiota contributes to the development of the immune system, intestinal homeostasis and host metabolism.1,2 Disruption of the gut microbiota is associated with a growing number of diseases.1,4,17–19,24–27
We identified several groups of intestinal bacteria that were differentially represented in infants born by cesarean delivery. Most striking, the Escherichia–Shigella lineage was underrepresented, which is consistent with a previous culture-based study that reported delayed colonization by E. coli.24 Also consistent with that report, we found that the phylum Bacteroidetes was undetectable in infants born by cesarean delivery. However, contrary to previous studies,5,24 we did not observe strong differences by mode of delivery in the prevalence of C. difficile or the relative abundances of Bifidobacterium or Clostridium.
Bacterial richness and diversity were lowest among infants born by elective cesarean delivery and highest among those born by emergency cesarean delivery. Although based on a small number of infants, these findings suggest that colonization of the infant gut may be affected differently by elective versus emergency cesarean delivery. Further research is warranted to determine whether these differences are related to feeding, antibiotics or perhaps “partial” microbial exposure during emergency cesarean delivery following membrane rupture.
Consistent with previous studies,22 our study detected lower bacterial richness and diversity in infants who were breastfed. This association is generally attributed to unique oligosaccharides found in breast milk, which serve as selective metabolic substrates for a limited number of gut microbes.28 We identified 2 bacterial families that were significantly overrepresented in the infants not receiving breast milk: Peptostreptococcaceae and Verrucomicrobiaceae. The family Peptostreptococcaceae includes C. difficile, a pathogen associated with enteric and atopic disease.25 This pathogen is more commonly detected in formula-fed than in breastfed infants,5 as was confirmed by our findings. We did not detect differential representation of the genus Bifidobacterium according to infant diet, as others have reported.22,23
It has been debated whether microbiota richness and diversity are clinically significant measures, or whether the prevalence of specific “beneficial” organisms is more important. We found the relative abundances of certain bacteria to be correlated with overall microbiota richness and diversity. These correlations were positive in some cases and negative in others, illustrating the complexity of this microbial ecosystem and reflecting possible symbiosis or competition between species. Further research is required to disentangle the biological relevance of individual organisms from that of more global diversity index measures.
Finally, similar to other sequencing-based studies,18,26 we observed high variability of gut microbiota between infants. This finding concurs with previous reports in which gut microbiota profiles varied widely in the first year of life, before eventually converging toward a more stable and adult-like microbiota.23 As others have reported,29 we detected a predominance of the genus Bifidobacterium in the gut microbiota of many infants. However, in our cohort, some profiles were dominated by Firmicutes, and several contained no Bifidobacterium species at all.
Strengths and limitations
The major strength of our study is the application of new high-throughput gene sequencing technology to characterize the gut microbiota of healthy infants. Our sample is representative of the Canadian infant population, with 25% born by cesarean delivery and 42% fed by exclusive breastfeeding at 4 months of age (national averages are 27% and 43%, respectively).10,30
The study was limited by a lack of longitudinal data and insufficient power for detecting differences according to antibiotic use, type of cesarean delivery or brand of infant formula. Also, we could not assess interactions between exposure variables, which are frequently interrelated (cesarean delivery is typically accompanied by prophylactic use of antibiotics and can affect breastfeeding success). In the years ahead, these deficiencies will be addressed by our ongoing analysis within the CHILD cohort, where longitudinal sampling of more than 2000 infants is anticipated and multivariable analyses will be possible. In the meantime, our subsample of 24 infants is relatively large among studies of infant gut microbiota, many of which have included fewer than 15 infants.18,22,23,29
Conclusion
The findings of this study advance our understanding of the gut microbiata of healthy infants and illustrate how this essential microbial community can be influenced by parent and physician decisions regarding mode of delivery and infant diet.
Still, much needs to be learned about the determinants of the infant gut microbiota and associated health outcomes. First, what constitutes a healthy or “ideal” microbiota? Are bacterial richness and diversity fundamentally important, or is it more critical to acquire specific bacteria in a particular combination? Second, what additional factors drive the colonization of the infant gut? Existing studies have rarely addressed the role of family structure, maternal diet, infections during infancy or the physical environment (e.g., pets and household chemicals). Finally, how does early establishment of the gut microbiota influence health and disease later in childhood? Recent reports have linked infant gut microbiota with subsequent development of atopic disease and obesity.24–27 We ultimately plan to explore all of these associations by leveraging the comprehensive exposure assessments and clinical outcome measurements in the CHILD national birth cohort. Our current results will inform these and other investigations of the infant gut microbiota, which together hold immense potential for advancing our knowledge of the human microbiome and its role in health and disease.
Acknowledgements
The authors extend sincere appreciation to all of the study families and the CHILD research teams. They also thank Yiye Zeng for help with the statistical analysis and Brenda Koster for bioinformatics support.
Footnotes
Competing interests: Allan Becker is an advisory board member for Merck, Novartis and AstraZeneca; his institution has received research grants from Merck and AstraZeneca. No competing interests were declared by the other authors.
This article has been peer reviewed.
Additional CHILD Study Investigators are listed at the end of the article.
Contributors: David Guttman, James Scott and Anita Kozyrskyj conceptualized and designed the study. Malcolm Sears and the CHILD Study Investigators contributed to the design and execution of the CHILD Study. Meghan Azad analyzed data and drafted the manuscript. Theodore Konya processed samples. Heather Maughan processed sequence data. Allan Becker coordinated the recruitment of participants and the collection of samples and data. All of the authors contributed to the interpretation of data, critically reviewed the manuscript for important intellectual content and approved the final version submitted for publication.
Funding: This research was funded by the Canadian Institutes of Health Research (grant nos. 85761 and 227312), and was supported by AllerGen NCE, the Killam Trusts and Alberta Innovates — Health Solutions.
Additional CHILD Study investigators: Ryan Allen (Simon Fraser University), Dean Befus (University of Alberta), Michael Brauer (University of British Columbia), Jeff Brook (Environment Canada), Michael Cyr (McMaster University), Edith Chen (University of British Columbia), Denise Daley (James Hogg iCAPTURE Centre), Sharon Dell (Hospital for Sick Children), Judah Denburg (McMaster University), Susan Elliott (University of Waterloo), Hartmut Grasemann (Hospital for Sick Children), Kent HayGlass (University of Manitoba), Richard Hegele (University of Toronto), Linn Holness (St. Michael’s Hospital), Michael Kobor (University of British Columbia), Tobias Kollmann (University of British Columbia), Catherine Laprise (Chicoutimi University Hospital), Mark Larché (McMaster University), Wen-Yi Wendy Lou (University of Toronto), Joseph Macri (McMaster University), Piush Mandhane (University of Alberta), Gregory Miller (Northwestern University), Red-wan Moqbel (University of Manitoba), Theo Moraes (Hospital for Sick Children), Peter Paré (University of British Columbia), Clare Ramsey (University of Manitoba), Felix Ratjen (Hospital for Sick Children), Bruce Ritchie (University of Alberta), Andrew Sandford (James Hogg iCAPTURE Centre), Jeremy Scott (University of Toronto), Frances Silverman (University of Toronto), Padmaja Subbarao (Hospital for Sick Children), Scott Tebbutt (James Hogg iCAPTURE Centre), Tim Takaro (Simon Fraser University), Patrick Tang (University of British Columbia), Teresa To (Hospital for Sick Children) and Stuart Turvey (University of British Columbia)