Abstract
Background: There are limited data about the effect of maternal influenza infection on fetuses and newborns. We performed a secondary analysis of data from the Mother’s Gift project, a randomized study designed to test the effectiveness of inactivated influenza and pneumococcal vaccines during pregnancy.
Methods: In the Mother’s Gift project, 340 pregnant women in Bangladesh received either inactivated influenza vaccine or 23-valent pneumococcal polysaccharide vaccine (control). This study was performed from August 2004 through December 2005. We performed a secondary analysis of outcomes following maternal influenza immunization during two periods: when influenza virus was not circulating (September 2004 through January 2005) and when influenza virus was circulating (February through October 2005). We assessed gestational age, mean birth weight and the proportion of infants who were small for gestational age.
Results: During the period with no circulating influenza virus, there were no differences in the incidence of respiratory illness with fever per 100 person-months among mothers and infants in the two groups (influenza vaccine: 3.9; control: 4.0; p > 0.9). The proportion of infants who were small for gestational age and the mean birth weight were similar between groups (small for gestational age: influenza vaccine 29.1%, control 34.3%; mean birth weight: influenza vaccine 3083 g, control 3053 g). During the period with circulating influenza virus, there was a substantial reduction in the incidence per 100 person-months of respiratory illness with fever among the mothers and infants who had received the influenza vaccine (influenza vaccine: 3.7; control: 7.2; p = 0.0003). During this period, the proportion of infants who were small for gestational age was lower in the influenza vaccine group than in the control group (25.9% v. 44.8%; p = 0.03). The mean birth weight was higher among infants whose mothers received the influenza vaccine than among those who received the control vaccine during this period (3178 g v. 2978 g; p = 0.02).
Interpretation: During the period with circulating influenza virus, maternal immunization during pregnancy was associated with a lower proportion of infants who were small for gestational age and an increase in mean birth weight. These data need confirmation but suggest that prevention of influenza infection in pregnancy can influence intrauterine growth.
Trial Registration: ClinicalTrials.gov: NCT 00142389
Influenza infection in young infants is common and results in high rates of hospital admission,1 but infection can be prevented by immunization of the mother during pregnancy.2 There are few prospective studies of the effect of antenatal vaccination against influenza on fetal and neonatal outcomes.3 There is conflicting information about the effect of maternal influenza infection on the fetus and newborn,3 though other antepartum maternal infections have well-described adverse effects on the fetus.4
The Mother’s Gift project is a randomized trial designed to assess the safety and efficacy of maternal pneumococcal and influenza immunization in Bangladesh. The primary outcomes of this study have been reported.2 In the current article, we report the results of a secondary analysis to assess the hypothesis that influenza immunization influenza the outcomes of infants whose mothers were exposed to influenza during pregnancy.
Methods
Study participants and design
This study was carried out from August 2004 through December 2005. The study design and primary analysis have been reported.2 Briefly, we randomly assigned 340 healthy pregnant women in the third trimester to receive either trivalent inactivated influenza vaccine (Fluarix; 2004, South hemisphere formulation) or the 23-valent pneumococcal polysaccharide vaccine (Pneumovax).2 Figure 1 provides the details of each study group. The women were interviewed at home each week from the time of immunization until six months after delivery. We recorded all respiratory illnesses, and infant illnesses were assessed by use of a rapid influenza antigen test.2
Flow of mothers and infants through the study. *Stillbirths occurred in December 2004, January and February 2005. There was no difference in the number of stillbirths between groups (Fisher exact test p = 0.3). Periods of influenza virus circulation: A) limited circulation, September 2004 through January 2005; B) confirmed circulation, February through October 2005.
The randomization sequence was generated by computer and was stratified by clinic and blocked in groups of four. Sequentially numbered opaque envelopes containing the study group assignments were provided to the recruitment clinics. Clinic nurses, who were not involved in the collection of project data, performed the immunizations. Mothers, families and study staff who collected illness and other data were unaware of the study group assignments.
Ethics approval
The protocol was approved by the institutional review boards of the International Centre for Diarrheal Disease Research, Bangladesh, and the Bloomberg School of Public Health at Johns Hopkins University. The use of commercial vaccines licensed by the US Food and Drug Administration was approved by the Directorate of Drug Administration of the Government of the People’s Republic of Bangladesh. Mothers and fathers provided signed informed consent.
Statistical analysis
Sample size
The primary goal of this study was to assess the immunogenicity of pneumococcal vaccine in mothers and infants, and influenza vaccine was chosen as the comparator.2 A sample size of 170 mothers and their infants in each group was chosen to provide adequate power for the primary outcomes of the study and to detect a 30% reduction of illness.2
Outcomes
The outcomes included in the present analysis were the proportion of infants who were small for gestational age (< 10th percentile weight for gestational age5), mean birth weight (as recorded in the delivery record), the proportion of infants with a low birth weight (< 2500 g), gestational age and the proportion of premature births (< 37 weeks gestation). We calculated gestational age from the reported first day of the last menstrual period. There is no Bangladesh-specific reference for weight for gestational age, so we used a North American standard.5 We also compared weight for gestational age using a global reference standard for growth developed by the World Health Organization; this standard was designed for use with full-term infants.6
Analysis
To compare outcomes between study groups, we calculated odds ratios (ORs) and we assessed differences in group means using the Student t test and the Mann–Whitney U test. We assessed proportions using the χ2 and Fisher exact tests. All tests were two-sided. We also calculated ORs for outcomes adjusted for selected characteristics (gestational age at vaccination, interval between immunization and birth) using multiple logistic and linear regression.
Classification of analytical study intervals
In our initial report,2 we used a rapid test to document the circulation of the influenza virus in Dhaka, Bangladesh, from January through October 2005. An independent local influenza surveillance study in Dhaka isolated influenza viruses in children with febrile respiratory illnesses from early 2004 through December 2007,7 and they reported very few influenza viruses among ill children between September 2004 and January 2005. Some influenza virus isolates were reported during February and March, and a substantial number of influenza A/H3N2 and B viruses were isolated from April through June 2005.7 We defined two periods: one with limited circulating virus (September 2004 through January 2005) and one when the influenza virus was shown to be circulating (February through October 2005) (Figure 2). The randomization of mothers was successful overall in producing demographically similar maternal study groups;2 nevertheless, we tested for maldistribution in each study period of several factors likely to influence the effect of the vaccine on the fetus, including gestational age at immunization and delivery, the interval between maternal immunization and birth, and infant Apgar scores (Table 1). We also tested for distribution of maternal age, parity, height and weight three to four months after delivery.
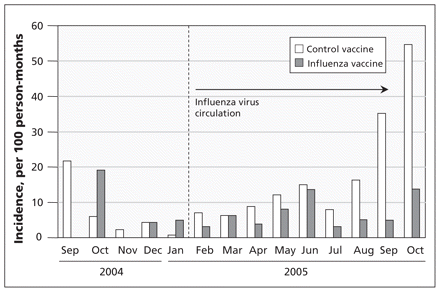
Incidence of respiratory illness with fever greater than 38°C among mothers and infants combined, and influenza virus before and after January 2005.
Characteristics of mothers, by study period
Our seasonal analysis focused on the 116 infants born during the February–June 2005 period who were in utero for 22–94 days after maternal immunization while the influenza virus circulated.
We created a binary variable for study periods before and after Jan. 31, 2005, to distinguish the period with limited virus circulation from the period with proven virus circulation. We performed a formal test of interaction, followed by an analysis stratified by the influenza circulation period. Subgroup analysis of clinical trial data are often discouraged to avoid misleading results, particularly in the absence of an independent underlying biological model.8 However, in studies of influenza virus infection and immunization, there is strong evidence for seasonality of the circulation of influenza virus and seasonality of antigenic matching of influenza vaccine with the circulating virus subtypes,9–13 providing substantial biologic support for this post-hoc analysis.
Results
During the study, 340 pregnant women were assigned to receive inactive influenza vaccine or pneumococcal vaccine (control) (Figure 1). In total, 336 infants were delivered, and 327 were included in our analysis. Three stillbirths occurred during the study period (December 2004, January and February 2005) among the mothers who received the influenza vaccine.
During the entire study period, 28% of infants in the influenza vaccine group were small for gestational age, and 38% were small for gestational age in the control group (OR 0.63, 95% CI 0.4–1.0; p = 0.05). The overall mean birth weight was 3% higher in the influenza vaccine group than in the control group (influenza vaccine 3117 g v. control 3027 g; p = 0.09) (Table 2).
Neonatal outcomes in overall study
During the period with limited virus circulation, there was no clinical effectiveness of the influenza vaccine on maternal plus infant respiratory illness (incidence rate ratio 0.99; p > 0.9) (Appendix 1 and 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110754/-/DC1). During the period with circulating influenza virus, the incidence rate ratio was 0.51 for all respiratory illness (p = 0.0003) (Table 3). There was significant interaction for respiratory illness between vaccine groups and the period (limited or confirmed circulating influenza virus) (p = 0.02).
Respiratory infections with fever greater than 38ºC among mothers and infants combined,* by influenza period and vaccine group
During the period with limited virus circulation, the proportion of infants who were small for gestational age was similar in both groups (influenza vaccine 29.1%; control 34.3%, OR 0.79 [95% CI 0.44–1.41]; p = 0.4) (Table 4). In contrast, during the period with circulating virus and increased clinical effect of the influenza vaccine (Figure 2), the proportion of infants who were small for gestational age was 25.9% in the influenza vaccine group and 44.8% in the control group (OR 0.43, 95% CI 0.20–0.94; p = 0.03) (Table 4). When we analyzed this data using the WHO growth standard, the OR was 0.39 (95% CI 0.16–0.92; p = 0.02) (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110754/-/DC1).
Neonatal outcomes, by study period
During the period with limited circulating virus, the mean birth weight was 3053 g in the influenza vaccine group and 3083 g in the control group (p = 0.9). In contrast, during the period with circulating influenza virus, the mean birth weight was 3178 g in the influenza vaccine group and 7% higher than 2978 g in the control group (p = 0.02) (Table 4, Figure 3).
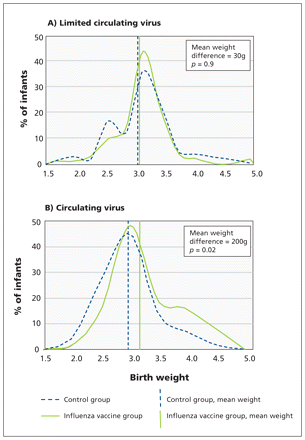
Distribution of birth weight by group and period. Periods of influenza virus circulation: A) limited circulation, October 2004 through January 2005; B) confirmed circulation, February through October 2005.
Figure 3 shows that, during the period with limited circulating influenza virus, the distribution of birth weight was similar in the two groups (Mann–Whitney U test, p = 0.9). In contrast, during the period with circulating virus, there was a shift in the distribution of birth weight to higher values among infants born to mothers vaccinated against influenza (Mann–Whitney U test, p = 0.02).
We did not detect a difference in the rate of premature births between the influenza vaccine and control groups when there was circulating influenza virus (3.5% v. 6.9%; OR 0.48 [95% CI 0.08–2.74]; p = 0.4) (Table 4). Although the number of infants with a low birth weight was small during the period with circulating influenza virus, there were fewer infants who weighed less than 2500 g in the influenza vaccine group (1.7%) than in the control group (8.6%); however, this difference was not significant (OR 0.19 [95% CI 0.02–1.64]; p = 0.09).
During the period with circulating influenza virus, the mean gestational age of the fetus when the mother was vaccinated was greater and the mean interval from immunization to birth was six days shorter in the influenza vaccine group than in the control group (Table 1), suggesting that, for the mothers who received influenza vaccine, there were fewer days available for the generation of a maternal immune response and for transplacental antibody transfer to the fetus, possibly limiting the observed effect of influenza vaccine for some study outcomes.
We performed multiple logistic regression to estimate adjusted ORs for small for gestational age after accounting for the effect of gestational age at immunization and the interval between immunization to birth. The adjusted ORs are similar to the unadjusted ones (Table 4). During the period with circulating influenza virus, the ORs were somewhat lower. Similarly, we performed multiple linear regression to estimate birth weight adjusted for gestational age at immunization and the interval between immunization and delivery. The adjusted birth weights were similar in magnitude to the unadjusted birth weights (Table 4). There were no significant differences between the influenza vaccine group and the control group in the distribution of infant gestational age at birth, Apgar score or the maternal characteristics of age, parity, height and postpartum weight (Table 1 and 2).
Interpretation
We found that immunization against influenza during pregnancy had a substantial effect on mean birth weight and the proportion of infants who were small for gestational age but only during the period of increased circulating influenza virus in the community.
Our data suggest that the prevention of infection with seasonal influenza in pregnant women by immunization can influence fetal growth. It has been reported that infection with pandemic influenza virus has substantial adverse effects in pregnant women and fetuses;14–18 however, there are limited similar data from epidemic seasonal influenza illness.3
The chronology of the specific effect of influenza vaccine on mean birth weight that we describe is distinct from the previously described general seasonality of birth weights in Bangladesh. Studies from Bangladesh have shown a seasonal pattern of reduction in the mean birth weights of full-term infants and an increase in the number of preterm infants in the season called monga, September to November, associated with reduced food availability before the annual harvest.19
There have been only four reports of infection of the fetus or placenta with nonpandemic influenza viruses, suggesting that this may be an infrequent event.20–23 The fetus gains 35–40 g/d (~1% of birth weight) in the last month of gestation.24 Several days of maternal illness, including fever, anorexia25 and circulating proinflammatory cytokines,26 even without severe maternal respiratory illness or admission to hospital, may account for the fetal effects we observed. The maternal generalized inflammatory responses to infection with the influenza virus likely modulates maternal metabolism, placental function and the transfer of nutrients to the fetus. The circulating maternal proinflammatory chemokines, cytokines and other factors27–29 may cross the placenta and have indirect inflammatory and metabolic effects in the fetus. The mechanisms of the effect of maternal influenza on fetal growth have not been described, and studies of the pathogenesis of this antenatal infection should be undertaken to elucidate approaches to treatment and prevention.
Several retrospective hospital record reviews and case–control studies have reported that infants born to mothers infected or exposed to influenza have mean birth weights similar to a variety of controls, but these observational assessments were not randomized trials30–33 and may not have captured the effects of influenza vaccine because of bias in the selection of cases or controls.
Several prospective randomized controlled trials of a variety of interventions for infectious illnesses in pregnant women have shown an increase in mean birth weight similar to the effect size we observed. For example, the use of malaria prophylaxis or antibiotic therapy in mothers with infections increased mean birth weight by 155–159 g.34,35 Randomized controlled trials of nutritional interventions in low resource settings have shown significant increases in mean birth weight, ranging from 70 to 200 g.36–39 A case–control assessment showed that prepartum pneumonia resulting in admission to hospital was associated with a 391 g reduction in mean birth weight.40
A recent retrospective observation of the effect of influenza vaccine in Georgia, United States, showed that the likelihood of small-for-gestational-age infants born to women who had been vaccinated against influenza was reduced by 69% during a period of widespread influenza activity.41 Several studies in North America and the United Kingdom of influenza-like ilnesses42 or proven influenza illness43,44 have reported significant reductions in mean birth weight, ranging from 90 to 285 g.
Strengths and limitations
The strengths of our study include the randomized double-blind design, the intention-to-treat analysis, the close observation of the participants and the monitoring of influenza infections in infants. The use of two growth standards with similar results provides some confidence about our observations. Because there is no Bangladesh reference for weight for gestational age, we used a North American standard.5 Many consider this reference population of a variety of ethnic groups, with relatively low levels of nutritional deprivation, to be a good reference for assessment of fetal growth potential.
Because the study outcomes were specified a priori, we did not correct for multiple comparisons in this analysis. Therefore, this secondary analysis may have some results that represent type I errors. In addition, the relatively small number of observations in 116 infants potentially exposed to maternal influenza infection during gestation may mean that some of our estimates are not precise.
We used clinical observations of maternal influenza-like illness, and we do not have supporting virologic data. The reduction of influenza-like febrile illness among the vaccinated women mirrored the reduction in laboratory-confirmed cases of influenza in their infants in the same household.2 The reduction of influenza-like febrile illness during periods with circulating influenza virus is accepted as a valid surrogate indicator for the effectiveness of influenza vaccine.10,13
The pneumococcal vaccine that was used as a control may have had an independent positive effect on the outcomes among infants. If that were the case, the observed difference between the two vaccine groups would be an underestimate of the true effect of influenza vaccine compared with placebo.
Our assessment of the effect of the influenza vaccine was carried out for about one year. This year may not be typical of every year, especially with respect to local circulation of several influenza virus strains in tropical regions.7
Conclusion
Our data suggest that influenza infection during pregnancy may affect fetal development. The overall absolute reduction in the proportion of infants who were small for gestational age was 0.1, suggesting that every 10 maternal influenza immunizations prevented the birth of one small-for-gestational-age infant. During the period with local circulation of influenza virus, the number needed to vaccinate to prevent one small-for-gestational-age birth is six mothers.
There is substantial data to show that full-term infants who are small for gestational age have an increased risk of lifelong consequences.45–47 Effective, safe vaccines to prevent influenza during pregnancy are available and recommended by a variety of agencies.48–51 Antenatal programs to vaccinate against tetanus in limited-resource regions have achieved a substantial level of coverage, reaching, for example, 80% of all pregnant women in Bangladesh.52 If our data about the effect of antenatal influenza immunization on fetal development are confirmed, the existence of effective antenatal immunization delivery systems suggests influenza vaccine may be a feasible addition to routine antenatal immunization programs.
Acknowledgements
The authors thank the field and data management team at the International Centre for Diarrheal Disease Research, Bangladesh, for their efforts. The authors thank Dr. David Sack for his invaluable assistance in the project.
Footnotes
Competing interests: Mark Steinhoff has served as a consultant for Merck. No competing interests declared by any other authors.
This article was peer reviewed.
Contributors: Mark Steinhoff had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Mark Steinhoff, Saad Omer, K. Zaman, Eliza Roy, Shams Arifeen and Robert Breiman designed the study. Eliza Roy, K. Zaman, Shams Arifeen and Rubhana Raqib acquired the data. Mark Steinhoff, K. Zaman, Eliza Roy, Caitlan Dodd, Saad Omer and Shams Arifeen performed the analysis and interpretation of data. Mark Steinhoff, Saad Omer and Caitlan Dodd performed the statistical analysis. Mark Steinhoff, Saad Omer, K. Zaman and Robert Breiman drafted the manuscript, which was critically revised by Mark Steinhoff, Saad Omer, K. Zaman, Eliza Roy, Robert Breiman and Rubhana Raqib. Shams Arifeen, K. Zaman and Mark Steinhoff provided administrative, technical or material support. K. Zaman and Mark Steinhoff supervised the study. All of the authors approved the final version of the manuscript submitted for publication.
Funding: The Mother’s Gift project was supported by the Bill and Melinda Gates Foundation; the United States Agency for International Development; Wyeth Pharmaceuticals Inc.; the Thrasher Research Fund; Aventis Pasteur; the International Centre for Diarrheal Disease Research, Bangladesh; and the Bloomberg School of Public Health at Johns Hopkins University.
Dr. Omer was awarded the Maurice R. Hilleman Early-Stage Career Investigator Award by the National Foundation for Infectious Diseases (NFID). The award was funded by an unrestricted educational grant to the NFID from Merck Co. Inc.; however, Dr. Omer had no direct interaction with Merck Co. Inc.