Abstract
Background: Most proton pump inhibitors inhibit the bioactivation of clopidogrel to its active metabolite. The clinical significance of this drug interaction is unknown.
Methods: We conducted a population-based nested case–control study among patients aged 66 years or older who commenced clopidogrel between Apr. 1, 2002, and Dec. 31, 2007, following hospital discharge after treatment of acute myocardial infarction. The cases in our study were those readmitted with acute myocardial infarction within 90 days after discharge. We performed a secondary analysis considering events within 1 year. Event-free controls (at a ratio of 3:1) were matched to cases on age, percutaneous coronary intervention and a validated risk score. We categorized exposure to proton pump inhibitors before the index date as current (within 30 days), previous (31–90 days) or remote (91–180 days).
Results: Among 13 636 patients prescribed clopidogrel following acute myocardial infarction, we identified 734 cases readmitted with myocardial infarction and 2057 controls. After extensive multivariable adjustment, current use of proton pump inhibitors was associated with an increased risk of reinfarction (adjusted odds ratio [OR] 1.27, 95% confidence interval [CI] 1.03–1.57). We found no association with more distant exposure to proton pump inhibitors or in multiple sensitivity analyses. In a stratified analysis, pantoprazole, which does not inhibit cytochrome P450 2C19, had no association with readmission for myocardial infarction (adjusted OR 1.02, 95% CI 0.70–1.47).
Interpretation: Among patients receiving clopidogrel following acute myocardial infarction, concomitant therapy with proton pump inhibitors other than pantoprazole was associated with a loss of the beneficial effects of clopidogrel and an increased risk of reinfarction.
Platelet activation and aggregation are key elements of the pathogenesis of acute coronary syndromes. Drugs that impair platelet function are an important part of treatment for patients with ischemic heart disease. Compared with acetylsalicylic acid (ASA) alone, the combination of clopidogrel and ASA significantly reduces the incidence of recurrent coronary events following acute myocardial infarction.1 The effectiveness of clopidogrel is underscored by evidence suggesting that delays in clopidogrel treatment, restricted access to the drug and premature cessation of therapy are associated with adverse cardiovascular outcomes.2–5
Clopidogrel is a prodrug that is converted in the liver to an active thiol metabolite, which irreversibly inhibits the platelet P2Y12 adenosine diphosphate receptor.6,7 This bioactivation is mediated by hepatic cytochrome P450 isoenzymes, with cytochrome P450 2C19 playing a major role.8 The activity of cytochrome P450 2C19 dramatically influences the antiplatelet effect of clopidogrel. Patients with loss-of-function polymorphisms have lower levels of the active metabolite of clopidogrel, diminished platelet inhibition during clopidogrel treatment and an increased risk of cardiovascular events relative to those without such polymorphisms.9,10
Given the important role of cytochrome P450 2C19 in the bioactivation of clopidogrel, drugs that inhibit this enzyme may reduce the antiplatelet effect of clopidogrel. Proton pump inhibitors are among the most widely prescribed medications worldwide, with more than 12.4 million prescriptions issued in Canada in 2004.11 Emerging evidence suggests that some proton pump inhibitors can inhibit cytochrome P450 2C19, possibly altering clopidogrel's pharmacokinetics and potentially leading to an increased risk of adverse cardiac outcomes.12–15 Among high-risk angioplasty patients treated with ASA and clopidogrel, use of omeprazole significantly reduced the antiplatelet activity of clopidogrel.14 In a series of patients taking clopidogrel, the risk of acute myocardial infarction was more than 300% higher among those who were highly adherent to proton pump inhibitors than among those not taking proton pump inhibitors.16 However, these studies included relatively few patients and had limited ability to adjust for potential confounders.
Recently published guidelines recommend proton pump inhibitor therapy for the majority of patients treated with ASA after acute myocardial infarction, many of whom will also take clopidogrel.17 Consequently, it is probable that millions of patients worldwide will receive combination therapy with a proton pump inhibitor and clopidogrel. However, the clinical significance of the potential interaction between these drugs is unclear. We sought to characterize whether the concomitant use of a proton pump inhibitor with clopidogrel was associated with adverse outcomes among older patients discharged from hospital after acute myocardial infarction.
Methods
Setting
We conducted a population-based nested case–control study among Ontario residents aged 66 years or older who were discharged from hospital between Apr. 1, 2002, and Dec. 31, 2007, after treatment for acute myocardial infarction. These individuals had universal access to hospital care, physicians' services and prescription drug coverage. The study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre.
Data sources
We examined the computerized prescription records of the Ontario Public Drug Program, which contains comprehensive records of prescription medications dispensed to Ontario residents 65 years of age or older. We identified hospital admissions using the Canadian Institute for Health Information Discharge Abstract Database, which contains detailed diagnostic and procedural information about hospital admissions. We used the Ontario Health Insurance Plan database to identify claims for inpatient and outpatient physician services. We obtained basic demographic information, including date of death, from the Registered Persons Database, which contains a unique entry for each Ontario resident who has ever received a health card. Researchers routinely use these databases to study drug safety,18–20 including the clinical consequences of drug–drug interactions.21–23 We linked the databases in an anonymous fashion using encrypted 10-digit health-card numbers.
Identification of patients and outcomes
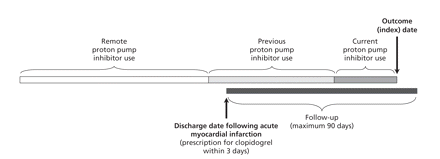
The study design is outlined in Figure 1. We established a cohort of patients aged 66 years or older who filled a prescription for clopidogrel within 3 days after hospital discharge following treatment for acute myocardial infarction. The date of discharge from hospital served as the date of cohort entry. We excluded patients who had received clopidogrel, ticlopidine or dipyridamole in the year before admission to hospital. We also excluded patients in long-term care facilities and those who received, within 90 days before or after the index date, proton pump inhibitor combination products used to eradicate Helicobacter pylori.
Figure 1: Study design. The solid black line indicates the period following hospital discharge after treatment of acute myocardial infarction. We excluded patients who did not receive a prescription for clopidogrel within 3 days of the discharge date (upward arrow). Among the remaining patients, cases were those readmitted because of myocardial infarction within 90 days after discharge. We attempted to identify 3 matched controls, with no myocardial infarction before the same date (the index date, indicated by downward arrow) for each case. Use of proton pump inhibitors before the index date was categorized as current (within 30 days), previous (31–90 days) or remote (91–180 days).
We followed patients who were receiving clopidogrel for a maximum of 90 days after hospital discharge or until readmission because of acute myocardial infarction. We identified patients with continuous use of clopidogrel using an adherence algorithm, which included patients with prescription refills of the drug at intervals not exceeding 1.2 times the number of days' supply of the preceding prescription. We identified hospital admissions using codes from the International Classification of Disease and Related Health Problems, 10th revision, for acute myocardial infarction (I21, I22). We designated the index date as the date of hospital readmission with acute myocardial infarction. For patients who had multiple readmissions with acute myocardial infarction during the study period, we considered only the first readmission.
We defined cases as patients who died or were readmitted for myocardial infarction within 90 days after the initial hospital discharge. We used random sampling with replacement24 from the same cohort of patients to identify the controls. These individuals were at risk but were not readmitted because of myocardial infarction before the index date. Controls were matched to cases on age (born within 3 years), receipt of percutaneous coronary intervention in hospital (defined as Canadian Classification of Procedure codes 1IJ50 or 1IJ57), date of hospital discharge (within 4 days) and predicted probability of short-term mortality (within 0.05 of that of the corresponding case), determined using a previously validated cardiac risk prediction model.25 When the number of controls who could be matched to a case was fewer than 3, we used only those controls and did not alter the matching algorithm.
Exposure to proton pump inhibitors
We used prescription records to ascertain exposure to proton pump inhibitors during clopidogrel therapy. We categorized use of proton pump inhibitors according to the most proximate prescription as either current (within 30 days before the index date), previous (31–90 days before the index date) or remote (91–180 days before the index date). We reasoned that a causal association between use of a proton pump inhibitor and recurrent myocardial infarction would manifest attenuating effects with more distant exposures.
Statistical analysis
We used conditional logistic regression to estimate the odds ratio (OR) for the association between reinfarction and exposure to a proton pump inhibitor, using as the reference group patients with no prescription for a proton pump inhibitor in the previous year. We adjusted for age, sex, income quintile (estimated from the residential postal code), Charlson comorbidity index,26 length of stay in hospital during the first admission for myocardial infarction, and 9 medical conditions previously shown to correlate with short-term mortality following acute myocardial infarction and identified at the index hospital admission (diabetes with complications, dysrhythmias, pulmonary edema, cardiogenic shock, acute renal insufficiency, chronic renal insufficiency, congestive heart failure and cerebrovascular disease.)25 We also adjusted for use of other commonly used cardiovascular medications, other cytochrome P450 2C19 inhibitors or inducers, and other cytochrome P450 3A4 inhibitors or inducers27 (Appendix 1, available at www.cmaj.ca/cgi/content/full/cmaj.082001/DC1) between hospital discharge and the reference date. In the secondary analysis examining use of histamine H2-receptor antagonists, we also adjusted for exposure to proton pump inhibitors between hospital discharge and the index date.
We conducted several additional analyses to test the robustness of our findings. We altered the definition of “current use” to include any prescription for a proton pump inhibitor between hospital discharge and the index date, and we conducted analyses of recurrent myocardial infarction and death within 1 year of hospital discharge. We examined use of the histamine H2-receptor antagonists ranitidine, famotidine and nizatidine as a tracer exposure. These drugs have clinical indications similar to those of proton pump inhibitors, yet they do not inhibit cytochrome P450 2C19. To test the specificity of our primary findings, we replicated our analysis in a cohort of patients who did not receive a prescription for clopidogrel within 90 days of discharge following acute myocardial infarction. Finally, we conducted a stratified analysis of the risk of recurrent myocardial infarction during treatment with pantoprazole or with other proton pump inhibitors. Pantoprazole does not inhibit cytochrome P450 2C19 and therefore should not interfere with the metabolic activation of clopidogrel, whereas other proton pump inhibitors or their primary metabolites do inhibit cytochrome P450 2C19 and can be expected to attenuate clopidogrel's beneficial effects.13
Results
Over the 69-month study period, we identified 13 636 patients aged 66 years or older who filled a prescription for clopidogrel within 3 days following hospital discharge after treatment of acute myocardial infarction. The median age of these patients was 76 (interquartile range 71–81) years; 7579 (55.6%) were men. The median length of stay during this admission was 5 days (interquartile range 4–8 days). A total of 4022 patients (29.5%) underwent percutaneous coronary intervention. Concomitant prescribing of proton pump inhibitors following discharge was exceedingly common, with 2682 (19.7%) patients receiving a proton pump inhibitor within 30 days of discharge and 4224 (31.0%) receiving a prescription within 90 days.
From this cohort, we identified 782 patients who were readmitted because of acute myocardial infarction within 90 days after discharge. Of these, 734 (93.9%) were matched to at least 1 control. The characteristics of cases and controls are shown in Table 1. As expected, cases were more likely than controls to have had several comorbidities during their index admission, including heart failure, diabetes and renal insufficiency.
Table 1.
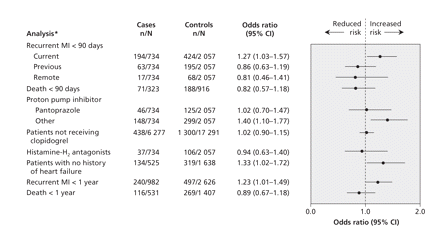
In the primary analysis, after extensive multivariable adjustment, we found a significant association between readmission because of myocardial infarction and current use of a proton pump inhibitor (adjusted OR 1.27, 95% confidence interval [CI] 1.03–1.57) but not with earlier use of these drugs. Our findings did not change appreciably when we redefined use of a proton pump inhibitor as a prescription between hospital discharge and the date of readmission, or when we included admission up to 1 year after the date of discharge (Figure 2). As expected, we found no association between recurrent myocardial infarction and use of histamine H2-receptor antagonists, and no such association among patients not treated with clopidogrel (Figure 2). In the stratified analysis of the type of proton pump inhibitor used, pantoprazole was not associated with recurrent myocardial infarction among patients receiving clopidogrel, as predicted by the observation that pantoprazole does not inhibit cytochrome P450 2C19.13 In contrast, compared with no treatment, other proton pump inhibitors were collectively associated with a 40% increase in the risk of recurrent myocardial infarction within 90 days of hospital discharge (OR 1.40, 95% CI 1.10–1.77) (Table 2 and Figure 2).
Figure 2: Association between acid-reducing therapies and adverse outcomes. Current use of proton pump inhibitors (within 30 days before the index date) was associated with recurrent infarction within 90 days and 1 year following hospital discharge after treatment of acute myocardial infarction (MI) among patients who were receiving clopidogrel. No such association was apparent with earlier therapy or among patients who were not receiving clopidogrel following acute MI. Treatment with histamine H2-receptor antagonists or pantoprazole, neither of which inhibits cytochrome P450 2C19, was not associated with recurrent infarction, whereas treatment with other proton pump inhibitors (omeprazole, lansoprazole and rabeprazole) was associated with reinfarction. Risk of death was not increased during therapy with proton pump inhibitors. *Data are for current proton pump inhibitor use unless stated otherwise.
Table 2.
We estimated the attributable risk of concomitant treatment with a cytochrome P450 2C19-inhibiting proton pump inhibitor and clopidogrel, using the point estimate of the adjusted OR (1.40) as an approximation of the relative risk, and with a conservative exposure estimate of 0.2 for any of omeprazole, lansoprazole or rabeprazole.28 Among older patients taking clopidogrel following acute myocardial infarction, we estimated that about 7.4% of readmissions because of reinfarction within 90 days after discharge occurred as the result of concomitant therapy with these agents. Using a less conservative estimate, if 40% of patients receiving clopidogrel were also prescribed one of these proton pump inhibitors, about 14% of all readmissions because of reinfarction could be attributed to this drug interaction.
Interpretation
In this population-based study spanning almost 6 years, we found that, among older patients taking clopidogrel following acute myocardial infarction, concomitant use of a proton pump inhibitor was associated with a significantly increased short-term risk of reinfarction. Depending on the prevalence of exposure to these drugs, we estimate that 5% to 15% of early readmissions because of myocardial infarction among patients taking clopidogrel could be the result of this drug interaction. In contrast, we found no association with more remote use of a proton pump inhibitor or current use of histamine H2-receptor antagonists. As predicted from its basic pharmacology, pantoprazole displayed no such association in a stratified analysis, whereas other proton pump inhibitors did. In sum, these observations support the hypothesis that some proton pump inhibitors significantly reduce or even abolish the cardioprotective effects of clopidogrel. Indeed, the increase in absolute risk of myocardial infarction during proton pump inhibitor therapy in this study approximated the 2.1% absolute risk reduction conferred by clopidogrel in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) study.1
Our findings have major implications for public health, given the number of patients exposed to this drug interaction. With annual sales of US$7.3 billion in 2007,29 clopidogrel is the drug with the second-largest sales volume worldwide. Millions of patients around the world receive a coronary stent or experience new or recurrent myocardial infarction each year. The majority of them will be prescribed clopidogrel in addition to ASA. Recent guidelines published by the American Heart Association, the American College of Gastroenterology and the American College of Cardiology advocate proton pump inhibitor therapy for the majority of patients receiving ASA after myocardial infarction, including all patients aged 60 years or older.17 Our findings suggest that indiscriminate treatment with a proton pump inhibitor could result in thousands of additional cases of recurrent myocardial infarction each year, all of which could potentially be avoided by preferentially using pantoprazole in patients taking clopidogrel who require treatment with a proton pump inhibitor.
Limitations
Some limitations of our research merit emphasis. We had no data on important cardiac risk factors such as smoking status, blood pressure and lipoproteins. Importantly, because our data sets included only drugs listed on the provincial formulary, we could not identify use of nonprescription medications, particularly over-the-counter ASA. Although we matched cases and controls on important predictors of outcome, some imbalance was evident in their measured characteristics (Table 1). However, this observation is expected and does not threaten our primary conclusion, because we found no association between proton pump inhibitor therapy and reinfarction in several sensitivity analyses, including an analysis of patients not receiving clopidogrel. Miscoding is a potential threat to the validity of any observational study, but previous validation studies have indicated that coding for acute myocardial infarction in Ontario's administrative databases is very good.30,31 Finally, because some patients take proton pump inhibitors intermittently, misclassification of exposure status is possible. However, with our study design, such misclassification would likely attenuate estimates of effect.
Conclusion
In summary, we found that, among patients taking clopidogrel following acute myocardial infarction, the concomitant use of a proton pump inhibitor that inhibits cytochrome P450 2C19 (omeprazole, lansoprazole or rabeprazole) was associated with an increased risk of recurrent myocardial infarction. This effect, which was not seen with pantoprazole therapy, presumably reflects inhibition of the metabolic bioactivation of clopidogrel. Our findings highlight a widely unappreciated, common and completely avoidable drug interaction in a population at high risk of recurrent coronary events. Pending further data regarding the clinical significance of drug interactions with clopidogrel, we believe that concomitant treatment with clopidogrel and proton pump inhibitors other than pantoprazole should be minimized when possible. Ranitidine or another H2-receptor antagonist may be an appropriate alternative for patients who require acid-lowering therapy. If a proton pump inhibitor is required, pantoprazole should be used preferentially in patients who are also receiving clopidogrel.
@@ See related commentary by Lau and Gurbel, page 699
Footnotes
-
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/180/7/713/DC1
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the study. Tara Gomes and Alex Kopp collected the data, and all of the authors contributed to the analysis and interpretation of the data. David Juurlink drafted the article. All of the authors revised it critically for important intellectual content and approved the final version submitted for publication.
Acknowledgements: We thank Donald Redelmeier for comments on an earlier draft of this manuscript, John Iazzetta for helpful comments and Ashif Kachra for assistance with manuscript preparation.
Funding: This study was supported by the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research (CIHR) and a CIHR Team Grant in Cardiovascular Outcomes Research. David Juurlink was supported by a New Investigator Award from the CIHR. Dennis Ko was supported by a Clinician Scientist Award from the Heart and Stroke Foundation of Ontario. Peter Austin was supported by a Career Investigator Award from the Heart and Stroke Foundation of Ontario. Jack Tu was supported by a Canada Research Chair in Health Services Research and a Career Investigator Award from the Heart and Stroke Foundation of Ontario. The opinions, results and conclusions are those of the authors. No endorsement by the Ontario Ministry of Health and Long-Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred.
Competing interests: Muhammad Mamdani was employed at Pfizer Global Pharmaceuticals from January 2006 to March 2007. No competing interests declared by David Juurlink, Tara Gomes, Dennis Ko, Paul Szmitko, Peter Austin, Jack Tu, David Henry or Alex Kopp.