Dilated cardiomyopathy is an uncommon complication of hypothyroidism.
Clinicians should consider dilated cardiomyopathy in patients with generalized pitting edema.
Cardiac dysfunction in patients with dilated cardiomyopathy secondary to hypothyroidism may be reversed with thyroid hormone replacement therapy.
Clinicians should consider evaluating thyroid function in patients with unexplained dilated cardiomyopathy, as some may have undiagnosed or untreated hypothyroidism.
A 45-year-old man presented to the general medicine clinic with a 2-week history of leg edema and dyspnea on exertion, including when walking up stairs. He had also noticed a new nonproductive cough. Five years before admission, his general practitioner had diagnosed hypothyroidism while ruling out secondary causes of dyslipidemia. He had been referred to our clinic, and we had diagnosed Hashimoto thyroiditis. His endocrinologist had treated him with 150 μg/d of levothyroxine; however, the patient was lost to follow-up 3 years later. He had no other relevant personal or family history, including autoimmune or cardiovascular diseases. He had smoked 20 cigarettes per day for 25 years, reported not consuming alcohol or illicit substances, and worked as an interior decorator. He was not on any medications at the time of admission.
On examination, the patient was conscious and alert, afebrile, slightly tachypneic (22 breaths/min), normotensive (116/88 mm Hg) and borderline tachycardic (100 beats/min). He had an oxygen saturation level of 99% on room air. On chest auscultation, we heard late inspiratory crackles in both lung fields and a third heart sound. He did not have raised jugular venous pressure.
We observed generalized pitting edema without overlying skin changes in the face, hands and lower legs up to the lower calves (Figure 1). In addition, we observed delayed relaxation of the ankle jerk reflex (Woltman sign) (Appendix 1, Video 1A, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220861/tab-related-content). Laboratory investigations showed a leukocyte count of 7200 (normal 3300–8600) cells/μL, hemoglobin level of 13.6 (normal 13.7–16.8) g/dL, creatine phosphokinase level of 786 (normal 59–248) U/L, creatine phosphokinase MB level of 6 (normal < 12.0) U/L, albumin level of 3.7 (normal 4.1–5.1) g/dL and brain natriuretic peptide level of 1140 (normal < 18.4) pg/mL. His thyroid profile included a thyroid-stimulating hormone level of 196.2 (normal 0.27–4.2) mIU/L, free thyroxine level of 1.80 (normal 11.58–23.17) pmol/L and free triiodothyronine level of 0.74 (normal 3.70–6.93) pmol/L.
Photograph of the right lower leg of a 45-year-old man with dilated cardiomyopathy, showing pretibial pitting edema (arrow).
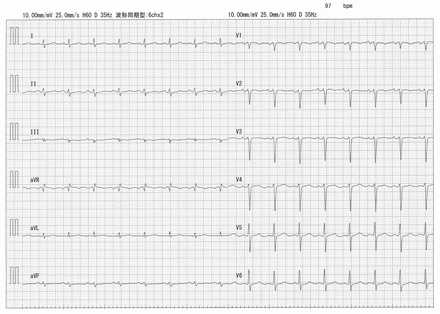
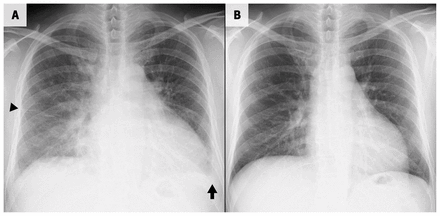
An electrocardiogram (ECG) showed slightly low QRS voltage in the limb leads and a biphasic P-wave in lead V1 (Figure 2). A chest radiograph showed cardiomegaly and pulmonary edema (Figure 3A). An ECG showed left ventricular global hypokinesis with a left ventricular ejection fraction of 28.4%, left ventricular enlargement with an end-diastolic diameter of 58.5 mm and an estimated right ventricular systolic pressure of 70.7 mm Hg. Cardiac magnetic resonance imaging (MRI) also showed left ventricular contractile dysfunction (Appendix 2, Video 2A and 2B, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220861/tab-related-content), with a left ventricular ejection fraction of 22.5% and right ventricular ejection fraction of 15.3%. However, we did not observe any late gadolinium enhancement or native T1 relaxation time changes on T1 mapping, with no evidence of myocardial edema and fibrosis.
Electrocardiogram trace of a 45-year-old man with dilated cardiomyopathy, taken on admission to hospital, showing slightly low QRS voltage in limb leads and biphasic P-wave in lead V1, with a corrected QT interval (Hodges) of 427 ms.
Chest radiographs (posteroanterior view) of a 45-year-old man with dilated cardiomyopathy. (A) Radiograph taken on the day of admission showing cardiomegaly, bilateral perihilar haze, small pleural effusion (arrow) and interlobular septal thickening (arrowhead). (B) Radiograph taken 2 months after discharge showing reduced cardiomyopathy and resolution of the pulmonary edema.
After discussions with the cardiology and endocrinology teams, and based on our patient’s emergent signs and symptoms, nonspecific ECG changes, biochemical evidence of severe hypothyroidism and absence of late gadolinium enhancement on cardiac MRI, we considered his presentation to be a consequence of severe untreated hypothyroidism. Our differential diagnosis included ischemic heart disease and exacerbation of chronic lung disease, given that our patient was a smoker (although he had no previous diagnosis of chronic obstructive pulmonary disease and had never sought medical attention for symptoms such as shortness of breath). We diagnosed congestive heart failure, secondary to dilated cardiomyopathy as a result of untreated hypothyroidism.
We treated the patient with oral furosemide (20 mg/d) and spironolactone (25 mg/d) for symptoms of heart failure. For his hypothyroid symptoms, we started treatment with a low dose of levothyroxine (6.25 μg/d), which we escalated weekly to avoid adverse cardiac events. We discharged him 22 days after hospital admission; he was on levothyroxine (37.5 μg/d) at discharge.
We reviewed the patient regularly as an outpatient. Two months after discharge, his symptoms had resolved fully, and a chest radiograph showed reduced cardiomegaly and no signs of pulmonary edema (Figure 3B). Therefore, we stopped the spironolactone and decreased the furosemide to 10 mg/d; we stopped it 2 months later. Three months after discharge, his delayed ankle jerk reflex relaxation had normalized (Appendix 1, Video 1B). By 6 months after discharge, the patient’s biochemical thyroid function had normalized when he was taking 150 μg/d of levothyroxine (Table 1). The patient’s left ventricular function also improved gradually, and 17 months after discharge, cardiac MRI showed improvement in cardiac contractility (Appendix 2, Video 2C and 2D); the left ventricular ejection fraction was 49.1% and the right ventricular ejection fraction was 56.0%. At 2-year follow-up, he was asymptomatic and able to work independently.
Investigations and levothyroxine dosage for a 45-year-old man with cardiomyopathy secondary to hypothyroidism
Discussion
Edema is a common symptom with several causes, including life-threatening diseases such as renal failure and heart failure. To identify the underlying cause, it is important to distinguish pitting from nonpitting edema on physical examination. Clinicians should assess the extent of edema, tenderness, and associated skin changes such as temperature, colour and texture.1 Nonpitting edema commonly occurs in patients with lymphedema and in those with hypothyroidism.1 It has been referred to as myxedema, which appears in peripheral areas such as the face or extremities, and sometimes presents as generalized edema.2
We initially thought our patient’s dyspnea on exertion was attributable to general fatigue, often seen in patients with hypothyroidism. However, dyspnea on exertion is also a common symptom of acute heart failure, which should therefore remain a differential diagnosis. Furthermore, nonpitting edema would be expected if all of our patient’s signs and symptoms were secondary to hypothyroidism only; however, he had generalized pitting edema, which prompted us to further investigate for evidence of heart failure.
Typical cardiovascular manifestations of hypothyroidism include bradycardia, pericardial effusion and narrow pulse pressure. 3,4 Hypothyroidism can lead to impaired cardiac contractility and diastolic dysfunction, which usually improve with thyroid hormone replacement therapy.4 However, the clinical course and imaging findings of our patient were more extreme, and he had developed dilated cardiomyopathy, characterized by left ventricular contractile dysfunction and dilatation. Although dilated cardiomyopathy is a common type of nonischemic cardiomyopathy, it is an uncommon cardiac complication of hypothyroidism, with only a few cases reported to date.4,5 Thus, epidemiological information is limited.3,5,6
The exact mechanism of the development of dilated cardiomyopathy in patients with hypothyroidism remains unclear. Thyroid hormones are known to mediate genomic and nongenomic effects on the heart, and recent studies have shown changes in gene expression associated with biosynthesis or diminished bioavailability of thyroid hormones at the tissue level among patients with dilated cardiomyopathy.7 Cardiac dysfunction among patients with dilated cardiomypathy secondary to hypothyroidism can be reversed with thyroid hormone replacement therapy.3,6 Although hypothyroidism alone is an uncommon cause of dilated cardiomyopathy, clinicians should perform thyroid function tests in patients with unexplained dilated cardiomyopathy.4
Adverse cardiovascular events may result from quick thyroid hormone replacement, without tapered escalation, especially among older patients, those with underlying ischemic heart disease or cardiac dysfunction, or those with severe and longstanding hypothyroidism.4,8 In our case, levothyroxine treatment (150 μg/d) was stopped 2 years before this presentation, and our patient subsequently developed ongoing heart failure with severe cardiac dysfunction. Although our patient did not have an underlying cardiac disease, we were concerned that he was at high risk of adverse cardiac events with aggressive hormone replacement therapy, and administered an initial low dose of levothyroxine that we titrated weekly under close follow-up. However, the “start low and go slow” approach is largely based on convention, and evidence supporting it is limited.4,8,9 It is uncertain whether patients with similar clinical characteristics as ours can tolerate quicker dose escalation, which would lead to earlier normalization of thyroid function.
We used cardiac MRI and echocardiography to establish a cardiac diagnosis and follow-up with our patient. Cardiac MRI is a promising modality for evaluating left ventricular function and following patients with dilated cardiomyopathy given its high degree of spatial resolution and reproducibility.10 Using a T1-mapping technique with cardiac MRI allows for quantitative evaluation of data, which is useful for assessing patients with diffuse myocardial diseases such as nonischemic dilated cardiomyopathy. 11 However, few cases of dilated cardiomyopathy secondary to hypothyroidism have been evaluated and followed using cardiac MRI. The usefulness of cardiac MRI in differentiating coronary artery disease from dilated cardiomyopathy has been reported.12
Patients with profound hypothyroidism may present with generalized edema and dyspnea, and clinicians should consider heart failure and cardiomyopathy as possible causes, in addition to the hypothyroidism itself. Dilated cardiomyopathy secondary to hypothyroidism is uncommon, but clinicians should perform thyroid function tests in patients with unexplained dilated cardiomyopathy; cardiac dysfunction may be reversed with thyroid hormone replacement therapy.
Videos showing the ankle jerk reflex and cardiac magnetic resonance imaging of a 45-year-old man with dilated cardiomyopathy secondary to hypothyroidism are available in Appendix 1 and Appendix 2, respectively, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220861/tab-related-content.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Naoki Matsuura drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/