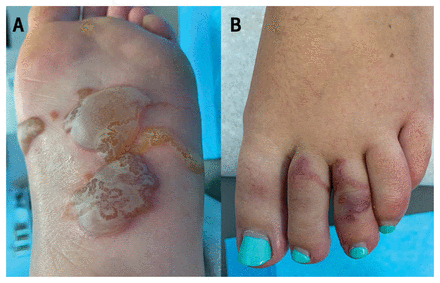
A 26-year-old woman presented to a clinic with tense bullae and associated migrating serpiginous tracks on her left foot (Figure 1) after returning from a 1-week vacation in Jamaica. During her trip, she noticed itching, beginning 1 day after walking barefoot on the beach. Nine days later, she was examined by her family doctor who diagnosed cutaneous larva migrans and prescribed oral mebendazole (100 mg twice daily for 3 days) and prednisone (50 mg/d for 5 days). Despite completing the treatment, she continued to develop new tracks and bullae and consulted a dermatologist 4 days later. She had no other symptoms. She was prescribed single-dose oral ivermectin (200 μg/kg), with follow-up 1 week later showing near resolution of the bullae and no new cutaneous tracks.
Photographs of the left foot of a 26-year-old female with cutaneous larva migrans. (A) Multiple tense bullae with overlying serpiginous tracts on the plantar surface of the left foot. (B) Serpiginous tracts along the dorsal aspect of the left toes.
Cutaneous larva migrans is a skin infestation caused by hookworm larvae found in infected dog and cat feces. Infection can occur after walking barefoot on contaminated sand or soil in a tropical region. Direct contact with the skin allows the larvae to penetrate the epidermis, leading to the formation of erythematous, edematous tracts and, occasionally, the development of associated vesicles and bullae, as in this case.1 Although the exact pathogenesis of bullae formation is unknown, hypotheses include a hypersensitivity reaction to an unknown larval antigen or epidermal cleavage owing to larval lytic enzymes.2 Diagnosis is based on history of travel to a tropical region and clinical examination showing migratory linear or serpiginous tracks. Serologic testing is not routinely recommended.3
Most cases resolve spontaneously in several weeks, but treatment with oral antihelmintic medications can reduce the duration of the infestation and decrease symptoms. First-line oral treatments include albendazole or ivermectin.3 Mebendazole has reduced bioavailability and less efficacy. Topical corticosteroids or antihistamines may be helpful adjuvants for symptom relief.
Footnotes
Competing interests: Ranjani Somayaji received research funding from the Cystic Fibrosis Foundation, Canadian Institutes of Health Research, University of Calgary and Vertex Pharmaceuticals. She has also received honoraria for speaking or been in advisory roles with Vertex Pharmaceuticals and Biomimir and has participated as a data and safety monitoring board member for Oncovir and the Cystic Fibrosis Foundation. Justin C. Chia received honoraria for speaking or has been in advisory roles for AbbVie, Celgene, Bausch Health, Eli Lilly, Galderma, Janssen, L’Oreal Group, LEO Pharma, Novartis, Pfizer, Sanofi-Genzyme, Sun Pharma and Union Chimique Belge (UCB). No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/