Recreational use of nitrous oxide is a growing problem in many jurisdictions, including Canada.
Although isolated, short-term use rarely leads to serious complications, chronic use can cause neurotoxicity that is often not fully reversible; cervical myelopathy, peripheral neuropathy and encephalopathy have been described.
The pathophysiology of nitrous oxide toxicity results from functional vitamin B12 deficiency.
Elevated homocysteine and methylmalonic acid are potential biochemical markers for the diagnosis, and magnetic resonance imaging and nerve conduction studies can help further define the presentation.
Cessation of nitrous oxide is the mainstay of treatment; supplementation with vitamin B12 and methionine are recommended.
Nitrous oxide (N2O) has become a popular but dangerous recreational drug. Colloquially referred to as “laughing gas,” it was first used therapeutically in 1844 for patients undergoing dental surgery.1 Although a weak anesthetic, it remains in use today, especially for pediatric and dental procedures.2 Its recreational use is now recognized as a growing problem in many jurisdictions, including Australia3–5 and several European countries,6 particularly the United Kingdom.7 The true prevalence of recreational nitrous oxide use in Canada is unknown. However, 10% of all respondents and 15% of Canadian respondents to the 2021 Global Drug Survey reported having used nitrous oxide in the preceding year.8 This Internet-based survey provides insight about patterns of drug use, but respondents are not representative of the general population. Although no Canadian agencies track nitrous oxide use, evidence of substantial recreational use is apparent in Toronto and Montréal.9,10 Large quantities of nitrous oxide and associated paraphernalia are easily ordered online, with rapid shipping to major cities.11 The drug’s popularity relates in part to its low cost, ease of access and perception of safety relative to other drugs.12,13
Although acute, heavy use of nitrous oxide can occasionally cause death by asphyxiation,14,15 isolated, short-term use rarely leads to serious complications.16 Regular inhalation, however, can have serious and even devastating neurologic consequences. We discuss recreational nitrous oxide use and its toxicity, including methods and patterns of use, pathophysiology, clinical presentation, diagnosis and management. We draw on evidence from case reports, case series, surveys and mechanistic studies related to nitrous oxide use and its complications (Box 1).
Box 1: Evidence considered in this review
We searched PubMed from 1956 to 2022 for case reports, case series, surveys and mechanistic studies related to nitrous oxide use and its complications. We supplemented this by screening bibliographies of these articles and of a leading textbook of toxicology (Goldfrank’s Toxicologic Emergencies16) to identify relevant articles regarding the pathophysiology, diagnosis and treatment of nitrous oxide complications.
What are the patterns of recreational use?
Most recreational users obtain nitrous oxide from cartridges of compressed gas intended for the preparation of whipped cream. Sometimes referred to as “Whippits,” these can be purchased in stores or online, often for less than $1 per 8-g canister. Its low cost and ready availability may play a role in the observation that nitrous oxide is the most commonly used inhalant in adults. The use of inhalants in general peaks around ages 13–14 years,17 with younger patients most often misusing solvent-based marking pens; however, the use of nitrous oxide peaks during early adulthood.18
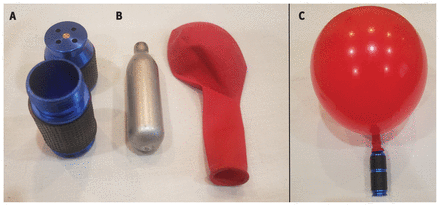
Using a whipped cream dispenser or a simple opening device called a “cracker,” users release aliquots of gas into a balloon, which is then used to deliver “hits” of the drug by inhalation (Figure 1).19 Some users inhale directly from dispensers or crackers, although this can cause cold-related injury to the mouth and skin, because the expansion of compressed gas is an endothermic process.20,21 Rarely, users will employ a face mask connected to a nitrous oxide source or a bag placed over the head; this poses a high risk of asphyxiation and is the primary cause of death from nitrous oxide.6,22
Apparatus for recreational nitrous oxide use. The nitrous oxide cartridge (B) is placed in a “cracker” (A), which punctures the cartridge, releasing the gas into the balloon (C).
Upon inhalation, users experience euphoria, analgesia and dis-inhibition. 13,19,23 The effects last only a few minutes, and repeated use is common when sustained effects are desired. Unlike therapeutic use, in which nitrous oxide mixed with oxygen is delivered in a monitored setting,24 recreational use of pure nitrous oxide carries the risk of alveolar hypoxia, when users attempt to achieve and sustain concentrations needed to produce euphoria.16,25 Regular users commonly use dozens of cartridges daily,19 with case reports describing use of more than 500 cartridges per day.26,27
What is the pathophysiology of nitrous oxide’s toxic effects?
The toxic effects of nitrous oxide result primarily from a functional deficiency of vitamin B12 (cobalamin) and are therefore chiefly neurologic and hematologic in nature. Vitamin B12 functions as a coenzyme for 2 important enzymes: methionine synthase and methylmalonyl coenzyme A mutase (MCM).
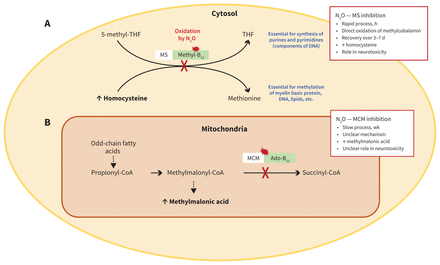
Under normal circumstances, methionine synthase converts homocysteine to methionine and 5-methyltetrahydrofolate to tetrahydrofolate. These 2 processes are coupled, relying on the transfer of a methyl group by vitamin B12 (as methylcobalamin; Figure 2). Nitrous oxide inactivates methylcobalamin by oxidizing its cobalt atom, effectively inhibiting methionine synthase. This impairs production of both methionine and tetrahydrofolate, which play key roles in the synthesis of myelin, as well as purines and pyrimidines (Figure 2). Nitrous oxide–induced neurotoxicity results primarily from impaired myelin synthesis, while megaloblastic anemia and other hematologic effects reflect the naturally high turnover of hematologic cells, a process that requires DNA and is therefore hampered by insufficient availability of purines and pyrimidines.24,28,29
Interaction between nitrous oxide (N2O) and vitamin B12 metabolic functions. (A) Methionine synthase (MS) converts homocysteine to methionine and 5-methyltehtrahydrofolate (methyl-THF) to tetrahydrofolate (THF). Methylcobalamin (the methyl form of vitamin B12) is an essential coenzyme in this process. Nitrous oxide quickly and irreversibly oxidizes the cobalt atom of methylcobalamin, rendering it inactive. The resulting inhibition of MS impairs folate activity and methionine synthesis, which are essential for DNA production and myelin integrity. It also leads to an increase in homocysteine. Methionine synthase inhibition largely underlies the neurotoxicity of N2O. (B) Methylmalonyl Co-A mutase converts methylmalonyl-CoA to succinyl-CoA, which then enters the Krebs cycle. Adenosylcobalamin (ado-B12; adenosyl form of vitamin B12) is a cofactor for methylmalonyl coenzyme A mutase (MCM). Prolonged use of N2O impairs the activity of MCM by unclear mechanisms. This increases methylmalonic acid concentration, which can be measured as a biomarker in patients with neurotoxicity secondary to N2O use. Note: white boxes = enzyme; green boxes = coenzyme of vitamin B12; red jagged circle = inhibition of enzyme activity; red “X” = interruption of the metabolic pathway.
Prolonged nitrous oxide use also inhibits MCM. In mitochondria, MCM catalyzes the conversion of methylmalonyl-CoA to succinyl-CoA, which then enters the Krebs cycle. The activity of MCM requires the coenzyme adenosylcobalamin (AdoCbl), a different form of vitamin B12 from that oxidized by nitrous oxide. The exact mechanism of MCM inhibition by nitrous oxide is unclear but may reflect the observation that oxidized methylcobalamin is more readily excreted, reducing overall stores of vitamin B12, mitochondrial AdoCbl and, subsequently, MCM activity in neural cells.30,31 The resulting accumulation of methylmalonic acid can serve as a sensitive diagnostic marker, but the extent to which MCM inhibition contributes to the clinical manifestations of vitamin B12 deficiency remains unclear.
Other complementary hypotheses to explain the neurotoxicity of nitrous oxide include N-methyl-D-aspartate (NMDA) antagonism,32,33 dysregulation of cytokines and growth factors that regulate myelin integrity,34 and hypoxia resulting from prolonged, heavy nitrous oxide use.35–37
What are the consequences of chronic nitrous oxide use?
The acute and chronic complications of recreational nitrous oxide use are summarized in Table 1.
Complications of recreational nitrous oxide use
Neurologic manifestations
Neurologic abnormalities are the most prominent features of chronic nitrous oxide exposure, with 3 well-described presentations. 22,28,38–40 The most commonly reported syndrome is myelopathy, generally in the form of subacute combined degeneration of the cervical spine. The second most commonly reported presentation is a peripheral sensorimotor neuropathy that predominantly affects the motor nerves.41 A third presentation is encephalopathy, which appears to be less common and is easily overlooked and often presents with new-onset psychiatric symptoms.
The distinction between myelopathy and peripheral neuropathy can sometimes be challenging, and patients with nitrous oxide neurotoxicity often exhibit both. They commonly present with bilateral paresthesia, weakness and gait disturbances. The lower limbs tend to be more severely affected, with isolated lower limb abnormalities found in as many as a third of patients.39 Common physical findings include reduced power and decreased sensation; pain and temperature sensation are more commonly impaired than vibration and proprioception, although abnormalities of both are frequent.41 Hypo- or hyperreflexia may be present, depending on whether peripheral nerves or the spinal cord are more prominently involved.39 Features of neurogenic bladder, such as urinary retention or incontinence, are present in a minority of patients. Other potential findings include ataxia, Romberg sign, Lhermitte sign and Babinski sign.22,38,39
Neurologic symptoms often develop acutely or subacutely after weeks to months of nitrous oxide use.28 Rarely, patients present after nitrous oxide anesthesia, with symptoms developing gradually in the weeks thereafter, mimicking the presentation of chronic recreational users.38,42 These patients tend to be older and many have marginal vitamin B12 stores at baseline, rendering them more susceptible to the effects of nitrous oxide.43–45 In a minority of cases, encephalopathy is present, with reported symptoms including paranoia, delusions, hallucinations and behavioural change.22,38,40,46
Dose-toxicity association
Unsurprisingly, neurologic complications are more common after repeated exposures. A survey of 16 124 recreational nitrous oxide users showed a strong association between the degree of exposure and the presence of neurologic symptoms.47 Among 76 regular users with neurologic symptoms, the median duration of use was 8 months, and the median exposure was 25 cartridges daily (interquartile range 8–85).38 However, patient factors such as baseline vitamin B12 status influence both the susceptibility to neurologic symptoms and the temporal course of their development. 38,48,49 Although no safe lower limit of exposure has been defined, complications after short-term use appear to be exceedingly rare. A recent systematic review found only 39 reported cases of neurotoxicity after nitrous oxide anesthesia.50,51
Other manifestations
Because nitrous oxide renders vitamin B12 nonfunctional, it can also cause hematologic abnormalities similar to those seen in pernicious anemia. Inhalation of 50% nitrous oxide for 1 hour can cause megaloblastic changes on bone marrow biopsy in susceptible patients, with similar features appearing after 12 hours of exposure in people with normal vitamin B12 stores.16,52 Macrocytic anemia is evident in 35%–50% of chronic users,38,39 while other features of vitamin B12 deficiency, such as leukopenia, hypersegmented neutrophils and thrombocytopenia, appear to be less common.52–55
Skin hyperpigmentation is also described,56 typically involving the dorsal aspects of the fingers and toes with maculopapular lesions on the trunk.57 In a series of 66 patients with nitrous oxide–related neurologic symptoms, dermatological findings were found in only 4 patients.58
Because impairment of methionine synthetase results in homocysteine elevation (Figure 2), a known risk factor for vascular disease, arterial and venous thrombotic events are theoretical complications of nitrous oxide use.59,60 No such effects were seen in the largest randomized controlled trial of nitrous oxide use in anesthesia,51 but these findings may not be generalizable to chronic users. A recent systematic review identified 14 reports of arterial or venous thromboembolism in young people with prolonged nitrous oxide use and no other obvious risk factors for thromboembolism.61 Most of these patients also had hyperhomocysteinemia.
What is the approach to making a diagnosis?
Nitrous oxide toxicity should be considered in the differential for all patients with signs and symptoms of peripheral neuropathy, myelopathy or encephalopathy, especially those who are younger. A history of nitrous oxide exposure, and heavy use in particular, is necessary to support the diagnosis, which highlights the importance of taking a comprehensive history of drug and substance use. When consistent clinical features and a history of heavy nitrous oxide exposure are both present, biochemical testing for functional vitamin B12 deficiency (homocysteine, methylmalonic acid) can confirm the diagnosis as described below (Table 2). Nervous system involvement should be further characterized with magnetic resonance imaging (MRI) and nerve conduction studies.28,38,50
Investigations for patients suspected of having nitrous oxide toxicity
Biochemical testing
A low vitamin B12 concentration is seen in 54%–72% of patients with neurologic complications from nitrous oxide exposure, 22,38,39 and is especially likely among those who develop symptoms after shorter exposures, presumably reflecting increased susceptibility.38 In chronic users, low vitamin B12 concentrations may reflect hastened elimination.31,34 Homocysteine and methylmalonic acid accumulate as a result of reduced enzymatic activity (Figure 2), and at least one is elevated in more than 90% of patients.38,62,63 Therefore, these markers are more sensitive than vitamin B12 concentrations, which remain normal in a substantial proportion of users despite neurotoxicity. Some users take supplemental vitamin B12 in an effort to prevent neurotoxicity, which can lead to normal levels of these biomarkers; this may falsely reassure clinicians but does not fully protect against the neurologic complications of nitrous oxide use.64–66
Imaging
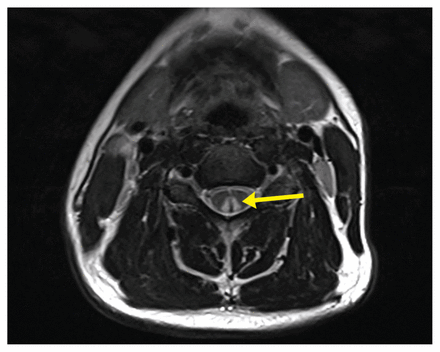
Magnetic resonance imaging of the spine is the preferred modality for the identification of myelopathy, revealing T2 hyperintensities in as many as two-thirds of patients with neurologic symptoms.38,39 A characteristic finding of subacute combined degeneration of the cord is the inverted “V” sign, corresponding to bilateral and symmetric T2 hyperintensities in the dorsal columns (Figure 3). This is typically most prominent in the cervical cord but can also be seen in the thoracic cord, in severe cases.38,39
Inverted “V” sign of subacute combined degeneration. T2 axial view of the spinal cord in a patient with extensive recreational use of nitrous oxide. Symmetric bilateral hyperintensities in the posterior cords (yellow arrow). Case courtesy of Jay Gajera, Radiopaedia.org, rID: 83938.
Although MRI of the brain is often normal, white matter changes are sometimes seen in patients with neuropsychiatric symptoms, typically involving the frontal lobes.67,68 In a series of 110 cases of symptomatic recreational nitrous oxide users, frontal lobe demyelination was evident in 3 of 11 patients who underwent brain MRI.39
Nerve conduction studies
Nerve conduction studies are abnormal in most symptomatic patients.22,28,39 The most common abnormalities are axonal degeneration, with or without demyelination, with isolated demyelination present in a minority of patients. In contrast to patients with quantitative vitamin B12 deficiency not caused by nitrous oxide, who tend to have more prominent sensory abnormalities, nitrous oxide users often exhibit more pronounced motor dysfunction.41
How should patients be treated?
The mainstay of treatment is cessation of nitrous oxide use. Vitamin B12 supplementation should also be given, sometimes in combination with methionine, although the evidence supporting efficacy is limited (Box 2).16 Given its favourable safety profile, we suggest intramuscular or subcutaneous injection of 1000 μg vitamin B12 daily for 1 to 2 weeks, followed by weekly doses of 1000 μg parentally or daily doses of 2000 μg by mouth, until resolution of symptoms.16,69,70 We also suggest supplemental oral methionine 1 g, 3 times daily (for at least 4–6 weeks or significant improvement of symptoms), which is likewise safe.40,48,67,71 Folate supplementation is unlikely to benefit the patient and should not be given before vitamin B12 repletion because of the potential for exacerbation of symptoms and delayed recovery.29,48,72 Physical rehabilitation and psychological and social supports may be required in selected cases.
Box 2: Treatment of patients with nitrous oxide toxicity
Cessation of exposure: Consider addiction medicine expertise and psychiatric, psychologic and social support
B12 (cobalamin): 1000 μg intramuscularly daily for 1–2 weeks, followed by 1000 μg weekly or 2000 μg oral daily until resolution of symptoms
Methionine: 1 g oral 3 times daily for at least 4–6 weeks or significant improvement of symptoms
Other: Rehabilitation for neurologic disabilities
Other: Do not administer folate before B12 supplementation
Prognosis
Although the prognosis is variable, most (95%–97%) patients display at least partial improvement, but more than one-third of patients admitted to hospital have residual neurologic symptoms even after months of treatment.22,39,40 The onset of improvement may be gradual, sometimes with little to no change during the first month of treatment but significant improvement in the months thereafter, reflecting the importance of sustained avoidance of nitrous oxide.28,39
Prevention
The public health response to nitrous oxide use is beyond the scope of this review.6 However, some actions can be taken by physicians to limit short- and long-term complications. To minimize short-term risks of nitrous oxide, we suggest counselling against the use of methods that risk asphyxiation (e.g., affixed masks and bags over the head)22 and inhalation directly from cartridges20,21 to reduce the risks of asphyxiation and thermal injury, respectively. Use during safety-sensitive activities like driving should obviously be discouraged.
The prevention of long-term complications requires stopping or at least reducing nitrous oxide use. In addition to education, some users may benefit from formal addiction medicine expertise, and psychological, social and peer support. In heavy users who do not promptly cease nitrous oxide, clinicians should consider suggesting prophylactic vitamin B12 supplements. This may delay the onset of symptoms and afford the patient time to reconsider the practice or seek treatment for substance use disorder.48 In such cases, it must be understood that neurotoxicity is well described despite use of B12 supplements, and that the only reliable way to prevent morbidity is cessation of nitrous oxide use.73,74
Conclusion
The low cost of and ease of access to nitrous oxide make it a popular recreational drug, especially among younger people. It can cause functional vitamin B12 deficiency and is an easily overlooked cause of neurologic abnormalities, typically myelopathy, peripheral neuropathy or encephalopathy, sometimes accompanied by hematologic abnormalities. Clinicians should enquire about nitrous oxide use in patients with unexplained findings suggestive of vitamin B12 deficiency or other compatible neurologic symptoms. Questions for future research are listed in Box 3.
Box 3: Unanswered questions
What are the optimal dose and duration of vitamin B12 and methionine for the treatment of nitrous oxide neurotoxicity?
Can individual risk of neurotoxicity after short- and long-term exposure be predicted?
What pathophysiologic processes underlie the quantitative vitamin B12 deficiency and inhibition of methylmalonyl coenzyme A mutase in patients with prolonged exposure to nitrous oxide?
Acknowledgement
The authors thank Steven Curry for helpful comments on an earlier draft of this manuscript.
Footnotes
Competing interests: David Juurlink has received reimbursement for travel costs for presentations and scientific meetings from the Canadian Institutes of Health Research, Stanford University and Texas Tech University Health Sciences Centre. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Both authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/