Abstract
Background: Screening programs for abdominal aortic aneurysm (AAA) are not available in Canada. We sought to determine the effectiveness and costutility of AAA screening in Ontario.
Methods: We compared one-time ultrasonography-based AAA screening for people aged 65 years to no screening using a fully probabilistic Markov model with a lifetime horizon. We estimated life-years, quality-adjusted life-years (QALYs), AAA-related deaths, number needed to screen to prevent 1 AAA-related death and costs (in Canadian dollars) from the perspective of the Ontario Ministry of Health. We retrieved model inputs from literature, Statistics Canada, and the Ontario Case Costing Initiative.
Results: Screening reduced AAA-related deaths by 84.9% among males and 81.0% among females. Compared with no screening, screening resulted in 0.04 (18.96 v. 18.92) additional life-years and 0.04 (14.95 v. 14.91) additional QALYs at an incremental cost of $80 per person among males. Among females, screening resulted in 0.02 (21.25 v. 21.23) additional life-years and 0.01 (16.20 v. 16.19) additional QALYs at an incremental cost of $11 per person. At a willingness-to-pay of $50 000 per year, screening was cost-effective in 84% (males) and 90% (females) of model iterations. Screening was increasingly cost-effective with higher AAA prevalence.
Interpretation: Screening for AAA among people aged 65 years in Ontario was associated with fewer AAA-related deaths and favourable cost-effectiveness. To maximize QALY gains per dollar spent and AAA-related deaths prevented, AAA screening programs should be designed to ensure that populations with high prevalence of AAA participate.
In Canada, about 20 000 people receive a diagnosis of an abdominal aortic aneurysm (AAA) annually.1 Males aged 65–80 years have an AAA prevalence 6 times higher than females of the same age.2 Smoking, family history of AAA, coronary artery disease, atherosclerosis, hypercholesterolemia, and hypertension are other known risk factors.3–6 Rupture of an AAA is often fatal, with pre-hospital and perioperative mortality rates of around 50%.7 Scheduled AAA treatments include open surgery or endovascular aneurysm repair, with mortality rates of 1%–5%. Timely detection of AAA is critical to allow prophylactic repair once the risk of rupture exceeds that of surgery. The Multicentre Aneurysm Screening Study randomized controlled trial (RCT) in the United Kingdom found a 42% reduction in AAA-related mortality over 13 years of follow-up after a one-time screening with ultrasonography among males aged 65–74 years.8,9 In total, 4 RCTs have shown that one-time AAA screening reduces risk of AAA-related death, rupture, and emergency repair among males aged at least 65 years.7 Among females, who were included in only 1 of 4 RCTs and accounted for 7% of the trial’s sample, no significant benefit from screening was shown.7 Although existing Canadian guidelines support AAA screening among males, they are inconsistent regarding screening among females.1,7,10 The Canadian Task Force on Preventive Health Care recommends one-time screening with ultrasonography for AAA among males aged 65–80 years (weak recommendation, moderate quality of evidence). 1 The Canadian Society for Vascular Surgery recommends one-time screening ultrasonography for all males aged 65–80 years (grade 1a [i.e., strong, high-quality] evidence) and for all females aged 65–80 years with a history of smoking or cardiovascular disease (grade 2c [i.e., weak, low-quality] evidence).7
Despite Canadian guidelines supportive of AAA screening, no provincial or territorial screening programs exist in Canada. In addition to uncertainty around the impact of AAA screening among females, one contributory knowledge gap may be a lack of contemporary evidence on the cost-effectiveness of screening. In Canada, screening programs often receive public funding, in part based on economic evaluations considered by health technology advisory bodies.11,12 Canadian cost-effectiveness analyses are outdated since they are based on data more than 15 years old and did not consider endovascular aneurysm repair, which has become more common than open AAA repair.13,14 Another contributory knowledge gap is the unknown prevalence of AAA in Canada. The impact of screening programs implemented internationally has been undermined by the lower-than-expected prevalence of AAA identified among people attending screening programs.15,16 A model-based economic evaluation, using contemporary data and explicitly considering the aforementioned knowledge gaps, can advance our understanding of the value of AAA screening in Canada. Therefore, we sought to determine the effectiveness and costutility of one-time ultrasonography screening for AAA in Ontario.
Methods
Study setting
In Ontario, the single-payer public health care system provides coverage for all AAA-related imaging (screening or follow-up), physician services, and hospital care. Repair for AAA is offered at 20 vascular centres across the province and performed as an inpatient procedure.
Study design, population, and outcomes
We developed a fully probabilistic Markov model to compare the outcomes and cost-utility of AAA screening versus no screening for males and females aged 65 years or 75 years. Outcomes included life expectancy (life-years), quality-adjusted life-years (QALYs), the frequency of AAA-related deaths, health care costs, the incremental cost-effectiveness ratio (ICER), and the number needed to screen to prevent 1 AAA-related death.17 We followed the Consolidated Health Economic Evaluation Reporting Standards (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230913/tab-related-content).18
Model structure
A fully probabilistic Markov model simulated a cohort of 1000 people over a lifetime. To address second-order parameter uncertainty, we drew 1000 independent samples of 1000 people each using different sets of parameter values sampled independently from their distributions in Table 1 and Table 2 to generate outcome distributions.
Prevalence and probability estimates for people in Canada
Utility and cost estimates for people in Canada
We defined an AAA as an aortic diameter of 3 cm or greater, divided into small (3.0–4.4 cm males; 3.0–3.9 cm females), medium (4.5–5.4 cm males; 4.0–4.9 cm females) and large (≥ 5.5 cm males; ≥ 5.0 cm females) AAAs, as clinically accepted.31 Follow-up consisted of ultrasonography every 3 years for small AAAs and every year for medium AAAs.31 Detected AAAs were allowed to grow at set rates. Once a large AAA was detected, the person was referred for scheduled open surgical repair or endovascular aneurysm repair. The decision for scheduled surgery occurred based on size (i.e., large AAA) since only a small proportion of AAAs require surgery because of symptoms other than rupture or rapid growth.32 Those with no AAA would not be rescreened.
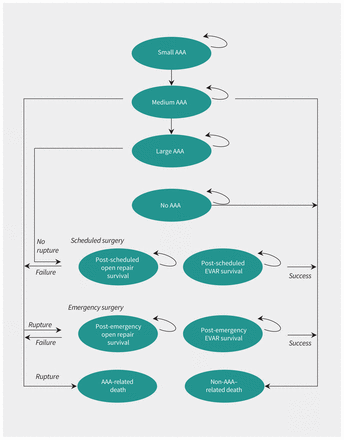
For each strategy, we modelled a person’s clinical course as time spent in 1 of 10 mutually exclusive health states (Figure 1). People could move from 1 state to another 4 times a year (3-mo cycle length). Unscreened people could only receive a scheduled repair after incidental AAA diagnosis. In case of rupture, a person would either die because of rupture before repair or undergo emergency surgery. Death could occur within any health state.
Markov model state transition diagram. Success and failure after surgery refers to survival and operative death, respectively. Note: AAA = abdominal aortic aneurysm, EVAR = endovascular aortic repair.
Model inputs
We derived model inputs from the literature, including RCT screening evidence, prevalence estimates from recent data from international screening programs, and operative outcomes from population-based studies in Ontario. When no data were available, inputs were based on our expert opinion. We performed costing from a health care public payer perspective (Ontario Ministry of Health) in 2022 Canadian dollars. Table 1 and Table 2 summarize model input parameters. Appendix 1 further describes model inputs and assumptions.
Base case analysis
Per the Canadian Agency for Drugs and Technologies in Health’s modelling guidelines, we discounted outcomes at 1.5% per year and applied a half-cycle correction.33 We developed incremental cost-effectiveness planes and cost-effectiveness acceptability curves to assess cost-effectiveness at a willingness-to-pay threshold of $50 000 per QALY and across a range of thresholds. We performed all analyses using TreeAge Pro 2021 (TreeAge Software).
Sensitivity analyses
We performed several 1-way and 2-way deterministic sensitivity analyses (Appendix 1). Because there was less uncertainty around surgery outcome estimates34,35 and those obtained from Statistics Canada,27,28 we focused on AAA prevalence, risk of rupture, growth rates, ultrasonography sensitivity and specificity, and follow-up compliance among those found to have an AAA. We modelled a higher AAA prevalence to better evaluate the Canadian Society for Vascular Surgery’s recommendation of screening females with a history of smoking or cardiovascular disease.7 In addition, we performed an alternative base case analysis for people aged 75 years, consistent with trial inclusion criteria and to align with Canadian guideline recommendations. Lastly, we performed deterministic sensitivity analyses for screening uptake and pre-hospital death to mimic real-life variations in adherence to preventive care services and accessing emergency surgical care in the event of rupture.
Validation
For external model validation, we calculated the expected number of avoided AAA-related deaths and compared this with published trials8,9,36–39 and non-Canadian decision analyses.19,21,34,40–42
Ethics approval
No ethics approval was required as all data were obtained from literature or publicly available data sources.
Results
The variables that were used as model inputs are shown in Table 1 and Table 2.
Base-case analyses
Compared with no screening, screening resulted in an additional 0.04 life-years (18.96 v. 18.92) and 0.04 (14.95 v. 14.91) QALYs, and an absolute reduction of 0.45% (0.08% v. 0.53%; 84.9% relative reduction) in AAA-related deaths among 65-year-old males. The incremental cost was $80 (ICER $2418 per QALY). Table 3 summarizes differences in outcomes. Table 4 compares findings to published models.
Base-case analyses comparing screening with no screening for abdominal aortic aneurysms (AAAs) over a lifetime
Country-level comparisons of population-based screening programs for abdominal aortic aneurysm among males aged 65 years
Compared with no screening, screening resulted in an additional 0.02 (21.25 v. 21.23) life-years and 0.01 (16.20 v. 16.19) QALYs, and an absolute reduction of 0.17% (0.04% v. 0.21%; 81.0% relative reduction) in AAA-related mortality among 65-year-old females. The incremental cost was $11 (ICER $740 per QALY).
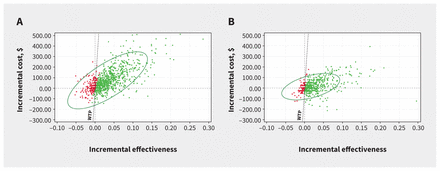
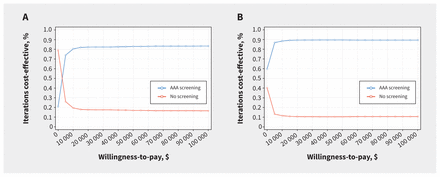
At a willingness-to-pay threshold of $50 000 per QALY, screening was cost-effective in 83.6% of model iterations for males and in 89.7% of iterations for females (Figure 2). Cost-effectiveness acceptability curves suggested that screening was cost-effective across a range of willingness-to-pay thresholds (Figure 3). The number needed to screen to prevent 1 AAA-related death is lower than for common cancer screening programs (Appendix 1, Appendix IV).
Incremental cost-effectiveness scatter plot for abdominal aortic aneurysm screening versus no screening among (A) males and (B) females aged 65 years in Canada. Bold dotted line represents the willingness-to-pay (WTP) threshold of $50 000 per quality-adjusted life-year. Costs were adjusted to 2022 Canadian dollars.
Cost-effectiveness acceptability curve for abdominal aortic aneurysm (AAA) screening versus no screening among (A) males and (B) females aged 65 years in Canada.
Alternative scenarios
For 75-year-old males, the net health benefit of screening versus no screening was 0.01 life-years (12.05 v. 12.04) and 0.01 (8.75 v. 8.74) QALYs. Screening resulted in an absolute reduction of 0.27% (0.07% v. 0.34%) in AAA-related mortality. The incremental cost was $107 (ICER $9697 per QALY).
For 75-year-old females, the net health benefit of screening versus no screening was 0.01 (13.97 v. 13.96) life-years and 0.00 (9.88 v. 9.88) QALYs. Screening resulted in an absolute reduction of 0.14% (0.03% v. 0.17%) in AAA-related mortality. The incremental cost was $16 (ICER $2349 per QALY). Table 3 summarizes differences in outcomes.
At a willingness-to-pay threshold of $50 000 per QALY, screening was likely cost-effective in 68.8% (males) to 82.8% (females) of model iterations. Cost-effectiveness acceptability curves showed that screening was likely cost-effective among males above willingness-to-pay thresholds of about $10 000 per QALY and among females at all thresholds (Appendix 1). The number needed to screen to prevent 1 AAA-related death remained lower than or comparable to common cancer screening programs (Appendix 1, Section II).
Sensitivity analyses
Deterministic sensitivity analyses showed screening was effective for all groups within reasonable ranges of key input parameters (i.e., pre-hospital deaths, ultrasonography sensitivity, ultrasonography specificity, and follow-up compliance); however, it highlighted that AAA prevalence had an important influence on net health gains (Appendix 1, Section III).
Interpretation
We found one-time ultrasonography screening for AAA among people aged 65 years in Ontario would reduce AAA-related deaths and would likely be cost-effective, with greater gains in life-years and QALYs among males. Screening remained likely to be cost-effective when considering people aged 75 years, lower screening uptake, and a lower rate of pre-hospital death in the event of rupture. Our modelling also suggested ways by which the cost-effectiveness of AAA screening could be strengthened. First, a focused AAA screening test using ultrasonography, reimbursed at a lower level than existing fee codes because of less extensive imaging, could result in cost savings. Second, participation in screening should be monitored and encouraged since cost-effectiveness is reduced with lower screening uptake. Third, AAA prevalence among screening participants should be monitored and populations at high AAA prevalence should be prioritized for participation, given more favourable cost-effectiveness. For example, with respect to females, screening those who smoke or have a history of cardiovascular disease — as suggested by the Canadian Society for Vascular Surgery recommendations — is reasonable.7
At 13-year follow-up, the UK’s Multicentre Aneurysm Screening Study found that screening among males aged 65–74 years was associated with a 42% risk reduction in AAA-related deaths and a 43% reduction in the number of ruptured AAAs.8,9 A Danish screening trial reported a 66% risk reduction in AAA-related mortality.38,39 A decision analysis for males aged 65 years in Canada, published in 2008, found comparably favourable results over a lifetime (QALY gain of 0.019, number needed to screen to prevent 1 AAA-related death of 137) but did not consider the effect of endovascular aneurysm repair.13 More recent international economic modelling among males aged 65 years supports our findings of cost-effectiveness. 21,40–42 More favourable cost-effectiveness for males in our analyses, relative to previous publications, may be explained by the better contemporary outcomes of scheduled AAA repair in Ontario, as documented in population-based analyses, and by improvements in life expectancy estimates relative to those used in older publications. We identified only 1 cost-utility analysis for females. Using data from the Multicentre Aneurysm Screening Study, a trial in which females made up only 7% of participants,7 Sweeting and colleagues34 found screening among females aged 65 years unlikely to be cost-effective (ICER GBP 30 000 [12 000–87 000] per QALY, given a willingness-to-pay threshold of GBP 20 000 per QALY). However, reduced mortality from an AAA was still confirmed. Our modelling, which used a lower AAA prevalence than seen in RCTs, confirmed the cost-effectiveness of screening among males and provides support for its cost-effectiveness among females, based on cost, life expectancy, and operative outcome estimates specific to the contemporary Canadian setting.
Our results should be placed in context of important considerations around AAA screening programs. The burden of screening on patients, such as logistics (e.g., transportation to screening and follow-up) or impact on mental health, are poorly studied for AAA screening programs implemented in other jurisdictions. Indeed, given the lower prevalence of AAA associated with reduced smoking, screening may impose proportionally greater harm; for every AAA-related death avoided, screening programs find 4 males with an otherwise undetected AAA that will never become symptomatic, resulting in overdiagnosis and psychological (and potential interventional) harms.43 This may especially be the case for females, who have lower AAA prevalence.44 Recent evidence from Sweden suggests that reductions in AAA deaths are likely due to reduced smoking rather than increased screening, questioning the benefit of continued population-based screening.16 Nevertheless, diagnosis of AAA through screening may have secondary cardiovascular benefits that may outweigh overdiagnosis, including detection of people at higher cardiovascular risk and initiation of secondary pharmacological prevention for other cardiovascular diseases.45,46 Another consideration of implementing AAA screening is that an increased volume of scheduled AAA repairs would add to existing wait lists. In 2018, patients in Ontario waited about 42 days between vascular surgeon consultation and receiving scheduled AAA repairs.14 Lastly, although more AAA-related deaths may be averted, compared with unnecessary lives lost during a scheduled repair (e.g., patient with AAA diagnosed through screening who would never have experienced a rupture but who dies during surgery), such a utilitarian lens may be acceptable only at the population level, rather than at the individual patient level.44 Thus, patients’ perspectives must be considered in developing screening programs. The decision to fund AAA screening programs at the provincial or territorial level may consider clinical evidence of efficacy, economic evaluations, patients’ perspectives, budget impact and equity and distributive justice.47 The scope and budgetary cost of an AAA screening program would also need to be considered in a budget impact assessment and implementation plan.
Limitations
Our results are sensitive to important parameters such as AAA prevalence rates. The true impact of screening programs in Canada will not be known until implemented. We modelled surgical complications as a homogeneous event, which oversimplifies the impact of different complications on health-related quality of life. Nevertheless, postoperative death is the most influential postoperative outcome, which we estimated using Ontario-specific population-based data. We did not account for differences by AAA risk factors, most notably smoking; however, we considered different thresholds of AAA prevalence. The growth of AAAs is difficult to predict and highly variable; as such, we simplified AAA sizing and used conservative growth rates, similar to published models.19,21,34,40–42 Screening programs are susceptible to healthy patient bias whereby healthier patients are more likely to attend screening programs, whereas patients with poorer health or challenging social determinants of health are less likely to attend screening. As a result, a lower prevalence rate may be observed among those screened than in the overall population, resulting in lower ICERs for screening programs. Despite these limitations, our model results were similar across multiple sensitivity analyses, show high face and external validity.
Conclusion
Our results support one-time ultrasonography screening for AAA in Ontario for males aged 65–75 years, consistent with current screening recommendations. Screening for females aged 65–75 years is likely cost-effective despite lower prevalence rates. Given our findings, the implementation of provincial AAA screening programs should be seriously considered. To maximize QALY gains and AAA-related deaths prevented per resources spent, AAA screening programs should be designed to ensure that populations with high prevalence of AAA participate.
Acknowledgement
This manuscript was reviewed and endorsed by the Executive Committee of the Canadian Society for Vascular Surgery.
Footnotes
Competing interests: Dominique Vervoort sits on the medical advisory board with the Global Alliance for Rheumatic and Congenital Hearts. Thomas Lindsay reports honoraria from Artivion and participation on an advisory board for Novartis. Varun Kapila is the provincial lead for vascular at Ontario Health. Charles de Mestral reports consulting fees from the Health Technology Assessment Unit, Ontario Health. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All authors made substantial contributions to study conception and design; acquisition, analysis, and interpretation of data; and drafting the work. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: Dominique Vervoort was supported by the Canadian Institutes of Health Research Vanier Canada Graduate Scholarship. This study was supported by the Bill and Vicky Blair Foundation Fund for Vascular Research.
Data sharing: All input parameters and assumptions are made available in the manuscript and appendix. The model structure can be made available upon reasonable request to the corresponding author.
- Accepted November 29, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/