Preterm premature rupture of membranes (PPROM), defined as rupture of the amniotic membranes before 37 weeks’ gestation, occurs in 2%–3% of pregnancies.
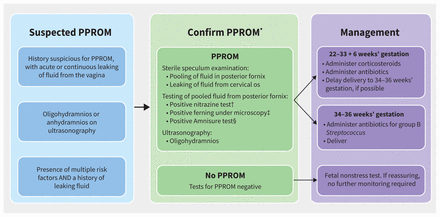
In patients with PPROM, a sterile speculum examination may reveal pooling of fluid that tests positive for high pH with nitrazine and for ferning under microscopy, as well as leakage of fluid from the cervical os; alternatively, AmniSure testing of the pooled fluid may be positive.
In addition to intravenous antibiotics (ampicillin 2 g and erythromycin 500 mg, every 6 h), Canadian guidelines recommend treating PPROM with 2 doses of intramuscular betamethasone (12 mg, 24 h apart) to accelerate fetal lung maturation.
Preterm premature rupture of membranes may lead to chorioamnionitis, shock (which can be delayed because of autotransfusion) and, uncommonly, severe cardiovascular complications.
Pelvic ultrasonography can rule out retained products of conception in postpartum patients who are not clinically improving after treatment with antibiotics for chorioamnionitis.
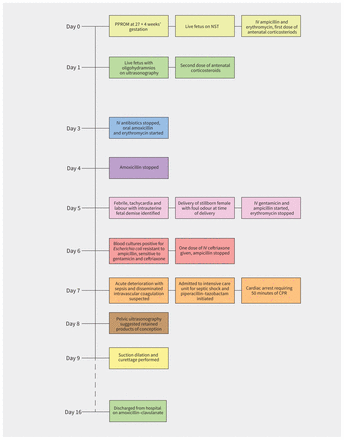
A 28-year-old woman with a history of 3 pregnancies and 2 live births presented to the labour and delivery unit of a district general hospital with preterm premature rupture of membranes (PPROM) at 27 + 4 weeks’ gestation (Figure 1). Membrane rupture was confirmed on sterile speculum examination; labour had not begun, and bleeding or decreased fetal movement were not observed. Maternal vital signs were normal, as was the fetal nonstress test, which measures fetal heart rate to assess well-being. The patient had no uterine tenderness. The pregnancy was otherwise uncomplicated. The patient’s 2 previous births were uncomplicated term vaginal deliveries. She also had mild asthma.
Timeline of clinical events. Note: CPR = cardiopulmonary resuscitation, IV = intravenous, NST = nonstress test, PPROM = preterm premature rupture of membranes.
In line with national guidelines for management of PPROM, the patient was admitted and treated with intravenous antibiotics (ampicillin 2 g and erythromycin 500 mg, every 6 h).1 She was prescribed 2 doses of intramuscular betamethasone (12 mg, 24 h apart) to accelerate fetal lung maturation. The next day, a live fetus and oligohydramnios was confirmed with ultrasonography. After 48 hours, as per national guidelines, the patient’s antibiotics were switched to oral amoxicillin (250 mg, every 8 h) and erythromycin (333 mg, every 8 h) for a further 5 days. Amoxicillin was stopped on day 4.1
On day 5, while an inpatient, the patient became febrile with painful contractions. Repeat ultrasonography was performed, which showed intrauterine fetal demise. The patient’s leukocyte count was 3.3 (normal 4.0–10.0) × 109/L, with neutrophils 2.9 (normal 2.5–7.0) × 109/L. Her labour was augmented with oxytocin, resulting in spontaneous vaginal delivery of a stillborn female, followed by a malodorous placenta, suggestive of chorioamnionitis. She was treated with ampicillin 2 g every 6 hours and gentamicin 340 mg (5 mg/kg) every 24 hours; erythromycin was stopped. The next day, cultures of blood samples grew Escherichia coli that was resistant to ampicillin but sensitive to gentamicin and ceftriaxone. Ampicillin and gentamicin were stopped, and she was treated with ceftriaxone 2 g every 24 hours.
On day 7, the patient developed chest tightness, in the absence of signs of deep vein thrombosis or pulmonary embolism. She deteriorated through the day, developing sepsis and suspected disseminated intravascular coagulation, and was admitted to the intensive care unit (ICU) for management of septic shock. Piperacillin–tazobactam was prescribed to replace the previous antimicrobial regimen.
Within 2 hours of transfer, cardiac arrest occurred, requiring 50 minutes of cardiopulmonary resuscitation. An extensive workup was carried out; imaging showed a subdural hematoma and a very small right-sided peripheral pulmonary arterial thrombus, which was deemed unlikely to be the cause of cardiac arrest, as well as bilateral pleural effusions and left lower lobe consolidation. Bilateral leg Doppler ultrasonography did not show thrombus. The patient’s electrocardiogram and echocardiogram were normal, excluding cardiomyopathy.
On day 8, the patient was extubated. She appeared neurocognitively intact. Her hypovolemia and cardiovascular status improved, but there appeared to be an ongoing untreated source of infection, despite treatment with broad-spectrum antibiotics. Pelvic ultrasonography suggested retained products of conception. Suction dilatation and curettage was performed on day 9 to remove these tissues. The patient subsequently improved and was discharged on day 16 on amoxicillin–clavulanate (875 mg by mouth, twice daily, for 3 d). Three weeks later, her echocardiogram was normal. At 3 months, her subdural hematoma had completely resolved. In an outpatient neurology clinic, the patient showed some deficits in concentration and immediate short-term memory, leading to challenges in performing discrete tasks and employment, but without other memory loss concerns.
Discussion
Preterm premature rupture of membranes, defined as rupture of the amniotic membranes before 37 weeks’ gestation, occurs in 2%–3% of pregnancies.2 Rupture results from weakening of the extracellular membrane of the chorion and amnion, often aggravated by infection ascending from the genital tract, most commonly caused by E. coli.3 This can lead to chorioamnionitis (infection of the placenta and membranes that surround the fetus). Risk factors for PPROM include amniocentesis, cervical procedures or insufficiency, previous PPROM or preterm birth, bacterial vaginosis or sexually transmitted infections (Appendix 1, Supplemental Table 1, https://www.cmaj.ca/lookup/doi/10.1503/cmaj.212068/tab-related-content).2
Preterm labour occurs spontaneously within 24 hours in 50% of patients with PPROM; 70%–80% deliver within 1 week of rupture.2 Signs of chorioamnionitis include fever, maternal tachycardia, abnormal fetal tracing, maternal leukocytosis, uterine tenderness and foul discharge at delivery.2
This patient had no identifiable risk factors for PPROM, nor any signs of intra-amniotic infection at the time of rupture. Given the gestational age of less than 34 weeks, and in the absence of evidence of infection, induction of labour was not indicated as the risks of prematurity outweighed the risks of infection with ongoing pregnancy (Figure 2). Induction may occur between 34 and 36 weeks’ gestation, depending on the balance of these risks; however, before 35 weeks’ gestation, induction should be delayed for at least 48 hours in the absence of infection to optimize the benefits of steroids.4 Daily monitoring for maternal and fetal well-being is recommended, including maternal vital signs and fetal nonstress testing while in hospital, usually for at least 48 hours to complete the PPROM intravenous antibiotic regimen.2
Algorithm for the confirmation of preterm premature rupture of membranes (PPROM) and its management, according to gestational age. Risk factors for PPROM are listed in Appendix 1, Supplemental Table 1, available at https://www.cmaj.ca/lookup/doi/10.1503/cmaj.212068/tab-related-content. *Not all tests are required for diagnosis of PPROM. Large-volume pooling or visualization of fluid leaking from the os, as well as a positive Amnnisure test, may be diagnostic on their own. Presence of ferning may be diagnostic alone, but is more reliable in combination with another test. Nitrazine is a nonspecific pH test of the pooled fluid in the posterior fornix obtained during a sterile speculum examination, and is not a reliable indicator alone. †Normal vaginal pH is 4.5–6.0 and amniotic fluid has a pH of 7.1–7.3. Nitrazine paper will turn from yellow to blue when a sample has a pH greater than 6.5.2 ‡Obtain a sample of pooled fluid in the posterior fornix on speculum examination and let it dry on a glass slide. With inspection under a microscope, a ferning pattern will be seen if the sample contains amniotic fluid.2 §Amnisure is a commercial test for samples of pooled fluid from the posterior fornix to indicate rupture of membranes based on detection of placental α-microglobulin (PAMGG-1).2
If patients with PPROM are discharged, they should complete 5 days of oral amoxicillin and erythromycin.1 Daily self-monitoring of maternal temperature is recommended.2 Evaluation for signs and symptoms of ascending infection (uterine tenderness, foul-smelling vaginal discharge), umbilical cord compression (decreased fetal movement) and umbilical cord prolapse (palpable or visible cord) are recommended. 2 Patients should also have weekly outpatient follow-up.
In cases where fetal presentation is not cephalic, hospital discharge should be balanced with the increased risk of cord prolapse. When managed expectantly between 24 and 27 weeks’ gestation, PPROM is associated with a 4.5% risk of stillbirth, which increases to 15% with chorioamnionitis.5 The unfortunate outcome of fetal loss in this case was likely secondary to infection.
When managing future pregnancies in patients who have experienced preterm birth from any cause, including PPROM, vaginal progesterone for prevention of preterm birth should be offered from 16–36 weeks’ gestation.6 This increases uterine quiescence and thickening of vaginal mucus, reducing the risk of ascending infection and pre-term birth.6
Maternal sepsis and cardiac arrest
Maternal sepsis is an important cause of obstetrical morbidity and death, with physiologic changes in the postpartum period potentially masking the expected hypovolemia of sepsis.7 Chorioamnionitis is the most common cause of pregnancy-related sepsis, and E. coli is the most common causal organism. Choriosepsis (progression of chorioamnionitis to sepsis) caused by E. coli is associated with the highest risk of intrauterine fetal demise and maternal ICU admission, compared with other pathogens such as group B Streptococcus and anaerobic bacteria.3,5 Our patient developed features of chorioamnionitis on day 5, including foul discharge at delivery and fever, but did not have any features on initial presentation with PPROM. Not all cases of chorioamnionitis show early signs or typical late signs. Clinical evidence of chorioamnionitis is present in 15%–25% of pregnancies complicated by PPROM, and an additional 51% have subclinical infection, detected only incidentally on histology.2
Maternal cardiac arrest is an uncommon complication in pregnancy and the postpartum period. One study showed that chorioamnionitis was found in 4.9% of maternal cardiac arrests, and sepsis from any source was present in 8.8% of maternal cardiac arrests.8 Unfortunately, 40% of cardiac arrests related to sepsis result in death.9
Ampicillin and gentamicin are standard therapies for chorioamnionitis. 10 Although gentamicin is appropriate for treating acute chorioamnionitis, it may not penetrate the infected retained products of conception. In our case, treatment with ceftriaxone would have provided improved coverage, but was administered only after a report of organism resistance to ampicillin. Patient response to Gram-negative lipopolysaccharides may also contribute to development of shock despite optimizing antibiotic coverage. Lipopolysaccharides bound to the outer membrane of Gram-negative bacteria are released into the patient’s circulation with antibiotic therapy, which may cause vasodilation and subsequent progression to circulatory shock.11 However, in our case, it is unlikely to have led to a 48-hour delay in hypotension, since endotoxin release plateaus about 10 hours after antibiotic exposure.12 Rather, this delay may be attributed to hemodynamic changes associated with postpartum autotransfusion.
Within the immediate postpartum period, autotransfusion can increase cardiac output by 60%–80%, with blood transferred from the uterus into systemic circulation.13 In our case, we hypothesize that postpartum autotransfusion may have transiently counterbalanced the typical hypovolemic state of sepsis over the first 24–48 hours. Coupled with lipopolysaccharide release, these phenomena made it difficult to identify sepsis and treat aggressively, thus leading to critical decline.
Lessons learned and recommendations
Patients with PPROM before 34 weeks’ gestation should be considered for transfer from the community to a tertiary care centre.2 Before transfer, community clinicians should obtain a complete blood count, monitor maternal vital signs, conduct a fetal nonstress test and administer the first dose of antenatal corticosteroids, ampicillin and erythromycin. 10 A maternal–fetal physician and a neonatologist should be consulted early.
In our case, although infection and cardiac arrest may not have been preventable, the patient’s clinical course underscores the importance of having tertiary care resources available in cases of PPROM, particularly before 34 weeks’ gestation. The Modified Early Obstetric Warning Score is an early sepsis recognition tool that may be helpful as it has good sensitivity, although specificity is low.14
For patients with signs of sepsis, involvement of infectious disease specialists and early communication with the intensive care team are recommended, even if transfer to ICU is not yet indicated, as clinical status may deteriorate rapidly.7 Interdisciplinary support is essential in postpartum management of fetal loss. Patients and their partners are at increased risk of complicated grief and should be offered counselling and support after stillbirth.2
Re-evaluation of the current protocol (ampicillin and erythromycin) to prolong pregnancy latency and prevent infection after PPROM may be warranted,1 given the increasing resistance of the usual causative pathogens to these antibiotics in Canada.14 A second- or third-generation cephalosporin (e.g., cefuroxime, cefoxitine, ceftriaxone), combined with a newer-generation macrolide (e.g., azithromycin), may more effectively prevent infection, as supported by a recent study.15
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Morgan Burgoyne and Naila Ramji drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/