Eosinophilic esophagitis is a chronic inflammatory disease of both children and adults, for which incidence and prevalence is rising worldwide; the condition is most common among young males with a history of atopy.
The pathophysiology involves an incompletely understood interaction of antigen exposures with host factors, including esophageal-specific genetic variations.
Adults with eosinophilic esophagitis commonly present with recurrent dysphagia and food bolus impaction, which may be masked by compensating behaviours, whereas children more often present with feeding problems, abdominal pain and vomiting.
Diagnosis of eosinophilic esophagitis is based on clinical history as well as eosinophilic-predominant inflammation on histological examination of biopsies taken at endoscopy.
First-line treatment may include pharmacologic agents or elimination diets, in conjunction with esophageal dilation if necessary to address and prevent food obstructions.
Eosinophilic esophagitis is a chronic condition, which can affect both children and adults and encompasses a spectrum of disorders; it is a common cause of esophageal dysphagia, esophageal narrowing and food impaction.1–6 Given its increased recognition in the past 3 decades,2 we discuss recent evidence related to eosinophilic esophagitis as well as recommendations about its management based on current consensus guidelines (Box 1).
Box 1: Evidence used in this review
We searched PubMed from 2013 to May 2023, concentrating on publications from 2018 onwards, when the diagnostic criteria of eosinophilic esophagitis were changed. Selected articles included guidelines, meta-analyses and randomized controlled trials.
Who gets eosinophilic esophagitis?
While eosinophilic esophagitis affects people of all ages, higher rates are seen among those aged 5–14 years and 20–45 years, and the prevalence is higher in cold climates.7 Males are 3–4 times more likely than females to develop the condition.1 A family or personal history of type 2 inflammatory disorders is common (e.g., asthma, atopic dermatitis).8–10 A reported 64-fold increased risk among brothers suggests heritability of the condition.11
Most incidence and prevalence estimates for eosinophilic esophagitis are derived from data on predominantly White populations. 7,12,13 North America and Europe have the highest reported incidence (5–20 new cases per 100 000 annually) and prevalence (9.5–58.9 adults per 100 000).12–15 A population-based study from Calgary found that, from 2004 to 2008, incidence rose from 2.1 to 10.7 cases per 100 000 and prevalence from 10.7 to 33.7 per 100 000.16 In the Castilla-La Mancha region of Spain, between 2011 and 2017, incidence was stable at 10 cases per 100 000, but prevalence rose from 44.6 to 111.9 per 100 000.17 Factors contributing to the apparent rising disease burden may include an increasing awareness of eosinophilic esophagitis and the evolution of diagnostic criteria.7,12–19
What is the pathophysiology?
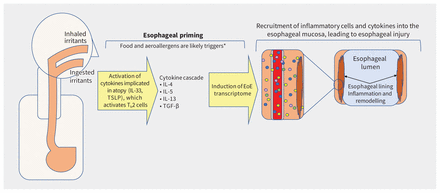
The pathophysiology of eosinophilic esophagitis involves an incompletely understood interaction of antigen exposures with host factors, including esophageal-specific genetic variations (Figure 1). Within families, genetic patterns suggest complex heritability. 11,20 Disruption of the esophageal epithelial barrier appears to be a trigger.3,20 Antigen exposure from food or the environment induces upregulation of cytokines implicated in atopy, which induce a cascade of inflammatory cytokines that stimulate the expression of a large group of genes called the eosinophilic esophagitis transcriptome.11,20 The subsequent migration of eosinophils and other inflammatory cells into the esophageal lumen wall causes an inflammatory cascade. Over time, this cascade contributes to transmural injury of the esophagus with development of fibrosis, narrowing of the esophageal lumen and stricture formation (Figure 1).21 The implicated genes overlap with those involved in allergic sensitization and type 2 immune disorders like asthma, atopic dermatitis and allergic rhinitis.11,20,22
Pathophysiology of eosinophilic esophagitis (EoE). Note: IL = interleukin, Th2 = T helper cells, TSLP = thymic stromal lymphopoietin. *Airborne triggers are not well established.
How do patients with eosinophilic esophagitis present clinically?
Dysphagia is the most common symptom of eosinophilic esophagitis. 1–3 Patients often present initially with a food bolus obstruction in the esophagus, usually along with the inability to swallow saliva.6,23 Patients may experience recurrent choking, belching or regurgitation due to real or perceived obstruction.24 Children are more likely to present with vague concerns such as abdominal pain, reflux, reduced appetite, chronic cough, failure to thrive, or vomiting.25 Most patients (75%) have at least 1 atopic condition, including food or environmental allergies, atopic dermatitis, allergic rhinitis, nasal polyps or asthma.8–10 Patients may also experience seasonal variability in their symptoms, with lower intensity in the winter months.26 Eosinophilic esophagitis is most often diagnosed in the summer, further underscoring the role of aeroallergen triggers.26,27
Adaptive eating behaviours and anxieties around meals are common. Patients may drink large volumes to assist in swallowing, cut food into small pieces and avoid foods with a hard texture. Mealtimes are drawn out and chewing can appear excessive. Many patients avoid taking pills. The IMPACT acronym is useful when taking a history (Box 2).3 Symptoms can be present up to 10 years before a diagnosis is made, increasing the risk of developing esophageal fibrosis.5
Box 2: The IMPACT acronym for taking a history when eosinophilic esophagitis is suspected
Imbibing fluids with meals to lubricate foods
Modifying food (cutting into small pieces)
Prolonged meal times
Avoidance of hard textured foods (e.g., bread, meats)
Chewing excessively
Turning away pills
How is the condition diagnosed?
The differential diagnosis for chronic esophageal dysphagia includes esophageal strictures induced by gastroesophageal reflux disease (GERD), esophageal motility disorders such as achalasia and eating disorders.
Differentiating between eosinophilic esophagitis and GERD can be difficult.1,28,29 The prevalence of esophagitis on endoscopy is similar (46% for eosinophilic esophagitis v. 56% for GERD).28 However, a retrospective case–control study identified predictors suggestive of eosinophilic esophagitis over GERD as young age, male sex, dysphagia symptoms, and food allergies.28 Absence of hiatal hernia, esophageal rings, furrows, plaques or exudates were more likely to be seen on endoscopy in eosinophilic esophagitis.28
To differentiate between eosinophilic esophagitis and motility or eating disorders, clinicians should look for red flags such as weight loss and malnutrition.30,31 The gold standard for diagnosis of a motility disorder is esophageal manometry, as endoscopic changes may be absent.32 Eating disorders may be difficult to differentiate from eosinophilic esophagitis since restrictive eating behaviours, including food aversions, anxiety around mealtimes and chewing behaviours, are a potential feature of both conditions. A detailed dietary history is essential to decipher which types of foods a patient avoids and why.30 Patients with eosinophilic esophagitis may avoid meat and bread because of previous choking episodes, while patients with eating disorders may do so because of caloric content.
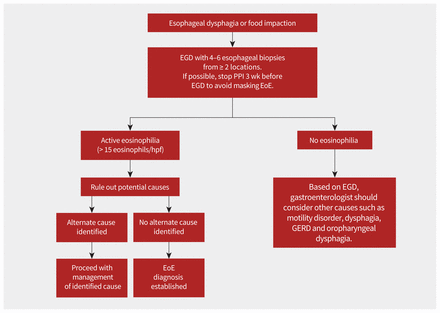
Diagnosis of eosinophilic esophagitis is based on both clinical history of esophageal dysfunction and esophageal biopsies showing eosinophilic-predominant inflammation on histology (≥ 15 eosinophils/high power field) (Figure 2).1–4,6 Appearance of the esophagus on endoscopy can vary and is not part of the diagnostic criteria.1,23 A trial of proton pump inhibitors (PPIs) was previously required, but this criterion was deemed unnecessary in 2018.2 Other potential causes of symptoms and esophageal eosinophilia should be excluded before making the diagnosis of eosinophilic esophagitis (Box 3 and Figure 2).1,2,4
Flow diagram to guide diagnosis of eosinophilic esophagitis (EoE). Note: EGD = esophagogastroduodenoscopy, GERD = gastroesophageal reflux disease, hpf = high power field, PPI = proton pump inhibitor.
Box 3: Medical conditions that can have eosinophilia on esophageal biopsies1
Eosinophilic esophagitis
Eosinophilic gastritis, gastroenteritis or colitis with esophageal involvement
Gastroesophageal reflux disease
Achalasia and other disorders of esophageal dysmotility
Hypereosinophilic syndrome
Crohn disease with esophageal involvement
Infections (fungal, viral)
Connective tissue disorders
Hypermobility sydromes
Autoimmune disorders and vasculitides
Dermatologic conditions with esophageal involvement (e.g., pemphigus)
Drug hypersensitivity reactions
Pill esophagitis
Graft v. host disease
Mendelian disorders (e.g., Marfan syndrome type 2, hyperimmunoglobulin E syndrome, PTEN hamartoma tumour syndrome, Netherton syndrome, severe atopy metabolic wasting syndrome)
A 2022 clinical guideline from the UK suggests stopping PPIs for a minimum of 3 weeks before endoscopy to avoid masking eosinophilic esophagitis,6 while other guidelines do not make such a recommendation (Figure 2).2,4,23 Biopsies should be taken at the time of endoscopy because 10%–32% of patients with eosinophilic esophagitis will have a normalappearing esophagus.1,23 Given the variable endoscopic and histologic appearance of eosinophilic esophagitis, 4–6 biopsy samples should be taken from at least 2 locations (proximal, middle and distal esophagus).1,4,23 Several studies have shown that diagnostic sensitivity increases with higher number of biopsies.23 The distribution of biopsies showing high numbers of eosinophils throughout the esophagus can help differentiate eosinophilic esophagitis from GERD in the context of other clinical factors. Eosinophilic esophagitis tends to affect multiple sites, while GERD usually shows eosinophilia in the lower sections of the esophagus. Once the diagnosis of eosinophilic esophagitis is confirmed, the gastroenterologist should follow-up with patients within a few weeks to discuss the diagnosis and commence treatment.
How is eosinophilic esophagitis managed?
Current guidelines recommend either pharmacologic or dietary treatment.4,6,23,33,34 Dilation may be required to manage esophageal narrowing from strictures and, in some cases, is done prophylactically to prevent esophageal obstruction. Together, these are the “3 D’s” (drugs, diet, dilation). To date, no head-to-head randomized controlled trials have established the superiority of any treatment modality. Small retrospective studies combining an elimination diet and medications have shown benefits among adults and children.6 Despite low-quality evidence,6 consensus suggests considering combination pharmacologic and dietary treatments in patients with limited response to single therapy, but this is still an evolving area of research.6,35
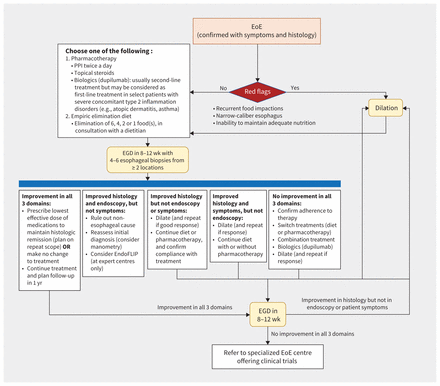
In accordance with current guidelines,4,6,23,33,34 we propose the algorithm in Figure 3. Early alignment of treatment options with patient preferences and expectations decreases patient frustration and increases adherence to therapy.3,4,6,33,35 The current evidence-based recommendation is to continue treatment once remission is achieved, as long as it remains acceptable to the patient.3,6,23,35 Further studies are required to evaluate long-term safety, sustained response to treatment over time and need for long-term maintenance therapy after remission or in mild disease.3
Proposed management algorithm for eosinophilic esophagitis (EoE) based on current guidelines and consensus statements, with consideration of the various outcomes of EoE care. Endoluminal functional lumen imaging probe (EndoFLIP) is a novel tool that studies esophageal wall stiffness and might have a role in fibrosis assessment in EoE. Note: EGD = esophagogastroduodenoscopy, PPI = proton pump inhibitor.
Pharmacologic therapy
First-line management is usually pharmacologic and can be started after obtaining esophageal biopsies on initial endoscopy (Figure 3).
Proton pump inhibitors are recommended as a first-line treatment.1,2,4,6,23,33 They are easy to use, are cost-effective and have relatively few adverse effects compared with other pharmacologic options.1,2,4,6,23,33 A systematic review and meta-analysis of 33 studies with a pooled population of 431 adults and 188 children found partial clinical and histologic responses to PPIs in 60.8% and 50.5% of the population, respectively.36 In a 2020 systematic review, taking PPIs twice daily induced histologic remission over 4–12 weeks in 41.7% of patients, compared with 13.3% of patients on placebo (relative risk [RR] 0.66, 95% confidence interval [CI] 0.61–0.72).33,34 Meta-analyses have categorized the evidence for the use of PPIs as very low quality, since more than two-thirds of studies were small retrospective case series or reports.36,37
Topical corticosteroids can be used in patients who do not respond to PPIs or as first-line therapy in patients with aggressive disease.1,2,6,33,34 A Cochrane review found that topical corticosteroids achieve clinical, histologic, and endoscopic remission, but they were not superior to PPIs.2,4,37–41 They reduce mucosal eosinophilia and stricture formation.1,3,6,23,33,34,40 For this reason, in our practice, we use topical corticosteroids as first-line therapy in patients with long-standing symptoms and a narrow esophagus, implying high risk of fibrosis and obstruction. No formulation has been shown to be superior in head-to-head comparisons.3,34,37,38 Topical corticosteroids are available as swallowed fluticasone propionate obtained from a nebulizer or as topical oral formulations (viscous budesonide or budesonide in an orodispersible tablet) (Table 1). The only formulation approved in Canada for adults (not children) with eosinophilic esophagitis is budesonide in an orodispersible tablet.39,40 A randomized controlled trial of budesonide (orodispersible tablet, 1 mg orally, twice daily) showed histologic, endoscopic and symptom improvement as early as 6 weeks in 58% of patients, compared with 0% of patients who received placebo.39 Sustained remission (48 wk) with budesonide (orodispersible tablet, 0.5 mg or 1 mg, twice daily) was seen in 73.5% who received the 0.5 mg dose and 75% of patients who received the 1 mg dose, compared with 4% of patients who received placebo.40 Median time to relapse was 87 days in the placebo group, compared with 350 days in the treated groups.40 The risk of oral candidiasis with topical corticosteroids is about 5%, so oral hygiene is important (Table 1).42
Pharmacologic management of eosinophilic esophagitis
Novel biologic agents targeting key drivers of type 2 inflammation have shown success in management of eosinophilic esophagitis (Figure 3).3,43,44 In May 2023, dupilumab (anti-interleukin [IL]-4/IL-13 monoclonal human antibody) was approved by Health Canada for the treatment of eosinophilic esophagitis. It was already approved for atopic dermatitis, chronic rhinosinusitis with nasal polyposis and asthma.43 Approval for eosinophilic esophagitis was based on a randomized controlled trial comparing dupilumab (300 mg weekly) with placebo,43,44 which showed histologic, endoscopic, and symptom improvement at 24 weeks (absolute risk reduction of 55%, 95% CI 40%–71%; p < 0.001).43 Sixty percent of patients in this study had previously used topical corticosteroids. After the initial 24-week period, the study had a 28-week open-label extension, whereby patients in the placebo group were given dupilumab weekly and those who initially received dupilumab continued to receive the same dose. After 52 weeks, patients who crossed over from placebo to dupilumab achieved similar remission rates (60%) to those originally on dupilumab (55.9%).44 Other monoclonal antibodies for atopic conditions, such as mepolizumab, benralizumab and omalizumab, have not shown benefit for eosinophilic esophagitis.6,33,34
Other medications — including systemic corticosteroids, leukotriene-receptor antagonists, cromolyn sodium, anti-immunoglobulin [Ig] E treatments, mercaptopurine and antitumour necrosis factor agents — have shown inconsistent results in several studies and are not recommended for treatment of eosinophilic esophagitis.4,6,33–35
Dietary modifications
Dietary modifications can help both to identify food triggers and to treat eosinophilic esophagitis (Figure 3).3,6,33,45 Empiric elimination and elemental diets are successful at inducing remission, but adherence is a challenge.45 Immunoglobulin E–based allergy skin and blood testing is not recommended for choosing the type of dietary restriction therapy because it has poor correlation with triggers, and is not a useful assessment tool for patients with eosinophilic esophagitis.6,45,46 Allergy-directed diets have an efficacy of 45.5% in achieving histologic remission when attempted for 2–12 weeks, although the results of studies evaluated in a meta-analysis had wide heterogeneity (I2 = 75%).46 Thus, these diets are not recommended.6,35
Empiric elimination diets avoid food groups commonly found to trigger atopic conditions. In studies on pediatric populations, 6 food allergen groups were implicated with eosinophilic esophagitis. The 6-food elimination diet removes cows’ milk, wheat, soya, nuts, seafood, and eggs to achieve esophageal healing. A systematic review of 10 observational studies comparing a 6-week course of the 6-food elimination diet versus placebo reported 68% versus 13% efficacy, respectively (RR 0.38, 95% CI 0.32–0.43).33,34
Eliminating 6 food groups is challenging, affects quality of life and may cause nutritional deficiencies.6,45 Support from an experienced dietitian is necessary when this is used in the treatment of eosinophilic esophagitis.45 Less restrictive elimination diets targeting dairy, eggs and wheat have been studied, as they account for about 50% of triggers.3,45,46 The 4-food elimination diet removes cows’ milk, wheat, and eggs, with the fourth food group being either soya or legumes.45 The 2-food elimination diet removes cows’ milk and wheat.45 Recently, a randomized, multi-centre open-label trial compared the 1-food elimination diet (cows’ milk) with the 6-food elimination diet and showed similar endoscopic, symptom and histologic remission (34% v. 40%, 95% CI –11 to 23; p < 0.58).47 Early involvement of a gastroenterologist and dietitian may increase the success of this treatment.6,35,45
Elemental diets have a limited role and are reserved for patients otherwise refractory to treatment.4,6 They are composed of amino acid–based liquid meal replacements. Proteins, fats and carbohydrates are broken down into amino acids, short-chain triglycerides and short-chain maltodextrins, and are combined with vitamins, minerals and electrolytes. Although effective, these diets are not popular choices for patients given their unpalatability, high cost and negative impact on quality of life.2,46
Dilation
In circumstances where diagnosis of eosinophilic esophagitis is delayed, fibrosis can develop and cause change to the caliber of the esophagus.5 Patients with symptoms of dysphagia may require dilation (Figure 3).6 Endoscopic dilation can be used to treat esophageal strictures and narrowing, reducing the risk of future food impactions, particularly among patients who have failed or not yet been treated with pharmacologic or dietary management (Figure 3). Patients with recurrent food impactions and a narrow-caliber esophagus (< 17 mm) on endoscopy should be offered dilation to reduce the risk of food bolus obstruction.23 Dilation relieves obstructive symptoms but does not treat the underlying inflammation; thus, it should be combined with anti-inflammatory therapies.3,23,35 In a study of patients requiring dilation, 65% of patients on pharmacologic or dietary treatment had a reduced need for repeat dilations after 2 years.23
Managing acute esophageal obstruction
Relief of acute esophageal obstruction by a food bolus can be attempted using noninvasive treatments. Drinking carbonated beverages may help to dislodge the impacted food.48 If the acute obstruction is not relieved with this measure, then the patient should have urgent endoscopy to provide relief. Glucagon, butyl scopolamine, calcium-channel blockers, nitrates and benzodiazepines were not found to be efficacious in several studies.48,49 Performing chest radiography before endoscopy was historically recommended to rule out esophageal perforation, but current guidelines do not recommend this because of the high false-negative rate.49
Even if a food obstruction resolves with noninvasive management, patients who present with food obstruction should be referred for outpatient endoscopy. More than 75% of patients will have an underlying cause of food obstruction, including eosinophilic esophagitis, strictures, motility disorders or malignancy.49 Lack of follow-up is a known predictor of recurrent food impaction.49
Patient follow-up
Patient symptoms and endoscopic and histologic findings do not always correlate with each other.23,35 After starting treatment, patients should be reassessed after 8–12 weeks, with consideration of both clinical symptoms and extent of esophageal healing, assessed by repeat endoscopy with biopsies (Figure 3).35,50 Efforts to identify less-invasive models of evaluating esophageal fibrosis are underway (e.g., the esophageal string test, Cytosponge, the endoluminal functional lumen imaging probe [EndoFLIP]), but their role is currently undefined.35 Medical therapy should be continued beyond symptomatic remission, and patient education around this concept is critical.35,50
Routine clinical and endoscopic follow-up should continue after remission is achieved, but an ideal timeline has not been established. 6,23,35 Guidelines and consensus publications now encourage maintenance treatment to avoid clinical and histologic relapse after stopping treatment.4,6,23,35,50 Figure 3 outlines how to manage the interplay of symptoms, endoscopic appearance and histology. Shared decision-making between the physician and patient is essential to mitigate the impact on quality of life from both the disease and its treatment (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230378/tab-related-content).6,8,24
A recent multinational effort to synthesize patient-, clinician-and researcher-recommended outcomes found that the most important markers of therapeutic efficacy were patient-reported symptoms, responses on eosinophilic esophagitis–specific quality of life questionnaires, histologic and endoscopic findings.51 A consolidated questionnaire, and the Index of Severity for Eosinophilic Esophagitis (I-SEE), is undergoing validation with the intent that a single tool will allow physicians to risk stratify, monitor and follow the disease across these domains over time.52
Conclusion
Eosinophilic esophagitis is a chronic disorder with a rising incidence and prevalence. The condition affects quality of life and can lead to esophageal strictures and fibrosis. Early detection and treatment with pharmacotherapy, dietary modification or endoscopic dilation can treat inflammation and reduce the risk of food bolus impactions. Shared decision-making is essential to the long-term success of eosinophilic esophagitis care. Questions for future research are listed in Box 4.
Box 4: Questions for future research
What are the specific triggers for inflammation in eosinophilic esophagitis?
Does early diagnosis and treatment improve long-term outcomes?
Are there different phenotypes of eosinophilic esophagitis that might guide specific treatment strategies?
How should refractory disease be treated? Is there an ideal combination of dietary and pharmacologic strategies?
What is the role of non-invasive techniques to detect and monitor disease?
How often should symptomatic patients be reassessed with endoscopy?
How often should patients with well-controlled symptoms undergo endoscopy?
Footnotes
Competing interests: Milli Gupta receives speaker fees from Sanofi Regeneron, AVIR Pharma, Bausch Health, Takeda and AstraZeneca, and participates on advisory boards for AVIR Pharma and Sanofi Regeneron. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Both authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/