Abstract
BACKGROUND: Although cancer incidence over time is well documented in Canada, trends by birth cohort and age group are less well known. We analyzed age- and sex-standardized incidence trends in Canada for 16 major cancer sites and all cancers combined.
METHODS: We obtained nationally representative population-based cancer incidence data in Canada between 1971 and 2015 from the National Cancer Incidence Reporting System (1969–1992) and the Canadian Cancer Registry (1992–2015). We analyzed cancer-incidence trends, reported as annual percent change (APC) for each 10-year group from age 20 to 89 years. We also estimated age-adjusted incidence rate ratios from fitted birth cohort models.
RESULTS: Across most age categories, the most recent trends show significant decreases in the incidence of cervical (APC −8.8% to −0.33%), lung (men: −7.42% to −0.36%; women: −6.27% to 1.07%), bladder (women: −4.12% to −0.07%; men: −5.13% to −0.38%) and prostate cancer (−11.11% to −1.11%). Significant increasing trends were observed for kidney, thyroid and uterine cancers. Overall incidence has increased among both sexes younger than 50 years of age, with recent increases in pancreatic cancer among men, breast cancer among women and colorectal cancer among both sexes. From the birth cohort analysis, we observed increasing trends in colorectal, liver and prostate cancers among men; kidney cancer and melanoma among women; and thyroid cancer among both sexes. We observed decreasing trends in cervical and ovarian cancers, and in bladder and lung cancers among men.
INTERPRETATION: Cancer incidence is decreasing at many sites targeted by primary-prevention efforts, such as smoking cessation and screening programs. Substantial increases in incidence among younger populations are driven by cancers possibly associated with obesity.
Examination of historical data in Canada suggests that overall age-standardized incidence rates of cancer have decreased in males, but have increased in females.1 Among both males and females between 1992 and 2013, the annual percent change (APC) in age-standardized incidence rates varied considerably among cancer sites, with the largest proportional increases seen in thyroid and liver cancers, and the largest decreases seen in stomach and laryngeal cancers.1 However, analysis of age-standardized overall cancer rates may mask important epidemiologic trends for a given cancer site. For example, while overall rates of colorectal cancer are decreasing in Canada, we have previously shown that incidence is increasing substantially among adults younger than 50 years of age.2 This discrepancy highlights the importance of examining the long-term trends in age-standardized incidence by individual cancer sites, which may be missed in overall trends.
Age-standardized trends may provide insights that are of clinical or epidemiologic value, such as how previous primary- and secondary-prevention interventions affected the health of a population. Analysis by birth cohort can also help to inform hypotheses about potential early-life risk factors that have changed over generations. Finally, understanding cancer-incidence trends specific to age and birth cohort can help guide future health-resource planning, as they may suggest health care needs of subpopulations. Although trends in cancer incidence have been well characterized in Canada for all ages combined, age- and birth cohort–specific trends have not been investigated for most cancer sites. In this study, we examined age- and birth cohort–specific incidence trends in Canada between 1971 and 2015 for 16 major cancer sites and all cancers combined.
Methods
We obtained population-based cancer incidence data in Canada from 2 nationally representative registries: the National Cancer Incidence Reporting System (NCIRS) (1969–1992)3 and the Canadian Cancer Registry (CCR) (1992–2015).4 The CCR evolved out of the NCIRS and is a longitudinal database of individuals in whom cancer was diagnosed, in contrast to the earlier event-oriented NCIRS, which recorded events of tumour diagnosis in Canada.5 Based on the Union for International Cancer Control global status of cancer registration, the CCR is one of the highest-quality national population-based cancer registries in the world.6
Complete cancer-incidence data were available in these databases for all provinces except Quebec, for which the most recent year of reported incidence data was 2010. Incidence data from 2011 to 2015 for Quebec were imputed for each sex–cancer site combination using the imputation method with the best overall performance (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1).
The starting year for analysis was 1971 because of the quality and availability of data from some provinces. We examined trends in age-adjusted (to the 2011 Canadian census population) population cancer incidence in 10-year age groups. For cancer sites and age groups with low average incidence (< 1 per 100 000 person-years), we used broader age categories. There was a change in reporting practices of bladder cancer in Ontario after 2012, at which point in situ cases were included. We used the jump model of the Joinpoint Regression Program, which was designed for the specific purpose of such changes.7 For the incidence of all cancers in Canada, we excluded nonmelanoma skin cancers, because most cancer registries do not collect incidence data on cases of nonmelanoma skin cancers. Discrepancies between the data sets from changes in cancer definitions over time were accounted for using methods that have been described previously.1 Briefly, from 1992 onward, cancer diagnoses were classified according to the International Classification of Diseases for Oncology, 3rd Edition. Before 1992, cancer diagnoses in the NCIRS were classified according to the equivalent codes from the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision. The NCIRS data were edited, reformatted and recoded to generate a standardized record file.8 Any changes in cancer definitions over time were reviewed and accounted for by the Health Sciences Division at Statistics Canada.9
Statistical analysis
We used the Joinpoint Regression Program (version 4.5.0.1, Surveillance Research Program, National Cancer Institute) to fit joined lines on a logarithmic scale to observed rates, and to estimate the APCs in incidence. We analyzed log-transformed incidence rates with permutation analysis to fit a series of joined straight lines, with a minimum of 0 and a maximum of 4 joinpoints. The best-fit model was selected by the Joinpoint Regression Program by performing a series of comparisons among the fitted models, including significance testing for identifying changes in trends. Detailed information is available on the program’s web page.10
We fit birth cohort models using the National Cancer Institute’s web tool.11 Input data were aggregated cases and population for 14 five-year age groups (20–24 yr to 85–89 yr) and 9 five-year periods (i.e., 1971–1975 through 2011–2015). Cohort effects by sex are presented as incidence rate ratios (IRRs), adjusted for age, with the 1941 birth cohort as the reference.
Sensitivity analysis
We conducted a sensitivity analysis excluding Quebec to determine robustness of our results.
Ethics approval
Ethics approval for this research was granted by the Health Research Ethics Board of Alberta (HREBA.CC-14-0220).
Results
For these analyses, 5 198 560 total incident cancer cases diagnosed between 1971 and 2015 were included (Table 1). Slightly more men (51.9%) than women (48.1%) were diagnosed at included cancer sites. Cancer incidence rose with each 5-year period from 277 540 cases in 1971–1975 to 880 385 cases in 2011–2015. More than half of all incident cancers were diagnosed in people aged 60–79 years. Cancer incidence by age, sex and cancer site are presented in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1).
Characteristics of included incident cancer cases (n = 5 198 560) in Canada from 1971 to 2015
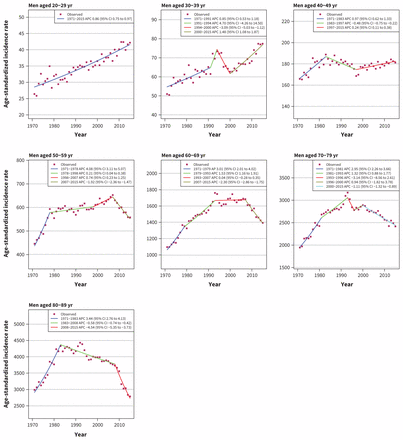
Overall, incidence of cancer among men is decreasing, which can be attributed to declines since 2007 among men older than 50 years (age 50–59 yr: APC −1.92, 95% confidence interval [CI] −2.36 to −1.47; age 60–69 yr: APC −2.30, 95% CI −2.86 to −1.75; age 70–79 yr: APC −1.11, 95% CI −1.32 to −0.89; age 80–89 yr: APC −4.54, 95% CI −5.35 to −3.73). Incidence has slightly increased among men in the younger age categories (age 20–29 yr: APC 0.86, 95% CI 0.75 to 0.97; age 30–39 yr: APC 1.48, 95% CI 1.08 to 1.87; age 40–49 yr: APC 0.24, 95% CI 0.11 to 0.38 (Figure 1).
Age-standardized incidence rates of all cancers in men in Canada (1971–2015). Note: APC = annual percent change, CI = confidence interval.
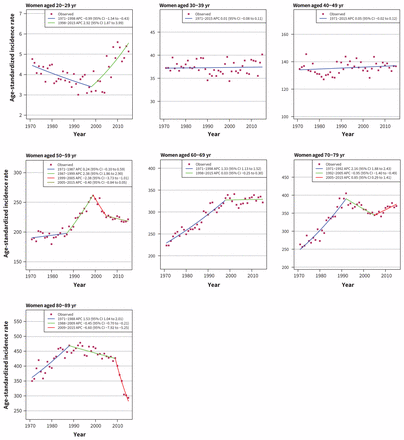
Among women, incidence is increasing for most age groups, with the highest rate of increase in women aged 30–39 years (APC 1.39, 95% CI 1.19 to 1.59). Cancer incidence has decreased among women aged 80–89 years, with an APC of −6.76 (95% CI −7.62 to −5.90) since 2009 (Figure 2). Trends for all selected cancer sites are summarized in Table 2.
Age-standardized incidence rates of all cancers in women in Canada (1971–2015). Note: APC = annual percent change, CI = confidence interval.
Age-standardized annual percent changes of the most recent trend* for selected cancer sites
Recent trends
We observed a decline in cervical cancer incidence, with significant trends observed in all age groups, except for the group aged 30–39 years, in which the recent change is not significant (APC −0.33, 95% CI −1.16 to 0.50). The declining trend starts after 1981 for the group aged 20–29 years (APC 1.22, 95% CI −0.98 to 3.47, before 1981), and after 1979 for women aged 80–89 years (APC 2.31, 95% CI −1.01 to 5.75, before 1979). For all other age groups, incidence was decreasing as of the start of incidence data in 1971 (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1).
Ovarian cancer has been decreasing among most age groups, with the exception of the group aged 20–29 years, in which a significant increase has been observed (APC 2.31, 95% CI 0.54 to 4.11), and in the group aged 40–49 years, in which rates have been stable recently (APC 0.70, 95% CI −0.06 to 1.46) (Appendix 3). Nevertheless, the incidence rates of these 2 age groups in 2015 were still similar to the rates in 1971 (age 20–29 yr: 2.26 per 100 000 in 1971 and 2.47 per 100 000 in 2015; 40–49 yr: 16.52 per 100 000 in 1971 and 13.33 per 100 000 in 2015).
Significant decreasing trends were observed for bladder cancer among all age groups in the most recent trend, with the exception of women age 70–79 years, in whom the trend was not significant.
We also observed significant increases in kidney cancer incidence across all age groups, except for groups aged 80–89 years, in which there has been a significant decrease for both sexes (men: APC −6.77, 95% CI −9.23 to −4.25; women: APC −7.43, 95% CI −10.96 to −3.76) (Appendix 3).
Uterine cancer incidence, according to the most recent trend, has been increasing significantly among women in all age groups, except for those aged 20–29 years, in whom incidence has been stable (APC 1.06, 95% CI −0.81 to 2.97), and in those aged 80–89 years, in whom incidence has been decreasing significantly (APC −2.26, 95% CI −2.82 to −1.70) (Appendix 3).
The incidence of liver cancer has been increasing among women older than 60 years, with sharp increases observed in more recent years in the group aged 60–69 years (APC 6.73, 95% CI 3.03 to 10.56). A steady increase in liver cancer incidence since 1971 was observed for most age groups among men, with APCs ranging from 0.95 (95% CI 0.14 to 1.77) in the group aged 20–39 years to 6.73 (95% CI 3.03 to 10.56) in the group aged 60–69 years. The exception was the group aged 40–59 years, in which a recent slight but nonsignificant decline has been observed (40–49 yr: APC −1.34, 95% CI −3.36 to 0.72; 50–59 yr: APC −1.62, 95% CI −3.68 to 0.49).
Melanoma incidence has also increased in all age groups, except in men aged 20–49 years (APC 0.05, 95% CI −0.16 to 0.26) and women aged 20–39 years (APC 0.21, 95% CI −0.02 to 0.44). In these groups, incidence rates have plateaued, and there has been a significant decline among men aged 20–29 years (APC −2.59, 95% CI −4.34 to −0.80) (Appendix 3).
There have been large increases in rates of thyroid cancer across all age groups, with more substantial increases observed since the 1990s (Appendix 3).
Top cancer sites among men: prostate, colorectal and lung
We observed a gradual increase in prostate cancer incidence rates among men aged 50–79 years between 1971 and the late 1980s (age 50–59 yr: APC 3.30, 95% CI 2.13 to 4.49; age 60–69 yr: APC 3.56, 95% CI 2.83 to 4.29; age 70–79 yr: APC 2.94, 95% CI 2.45 to 3.42), followed by a sharp increase between 1989 and 1993. In the age groups of 50–59 years and 60–69 years, the incidence rate kept rising until 2007 (age 50–59 yr: APC 5.18, 95% CI 4.31 to 6.06; 60–69 yr: APC 2.49, 95% CI 1.62 to 3.37), followed by a sharp decline afterward (50–59 yr: APC −6.32, 95% CI −7.61 to −5.01; 60–69 yr: APC −6.41, 95% CI −7.42 to −5.38). In the age group of 70–79 years, the incidence rate quickly dropped to the level before the sharp rise in 1996, remained steady between 1996 and 2007 (APC −0.68, 95% CI −1.38 to 0.02) and declined at an APC of −5.35 (95% CI −6.32 to −4.37) after 2007. In the age group of 80–89 years, the incidence rate gradually increased between 1971 and 1993 (APC 2.06, 95% CI 1.75 to 2.37). After 1993, the incidence rate decreased from 1423 per 100 000 in 1993 to 438 per 100 000 in 2015 (Appendix 3).
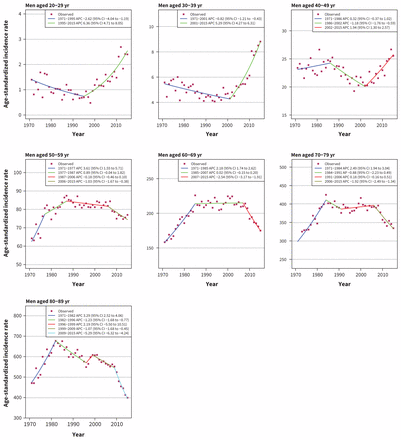
Incidence of colorectal cancer has increased in men younger than 50 years and decreased in men older than 50 years (Figure 3). The most recent trend (up to 2015) shows increasing rates for men aged 20–29 years (APC 6.36, 95% CI 4.71 to 8.05), 30–39 years (APC 5.29, 95% CI 4.27 to 6.31) and 40–49 years (APC 1.94, 95% CI 1.30 to 2.57), and decreasing rates for men aged 50–59 years (APC −1.03, 95% CI −1.67 to −0.38), 60–69 years (APC −2.54, 95% CI −3.17 to −1.91]), 70–79 years (APC −1.92, 95% CI −2.49 to −1.34) and 80–89 years (APC −5.29, 95% CI −6.32 to −4.24). Lung cancer incidence among men is decreasing in all age groups, with the largest decreases in the group aged 40–49 years (APC −7.42, 95% CI −9.02 to −5.79). No distinct trend could be discerned in the group aged 20–29 years, as incidence rate is very low, ranging from 0 to 0.7 per 100 000 (Appendix 3).
Age-standardized incidence rates of colorectal cancer in men in Canada (1971–2015). Note: APC = annual percent change, CI = confidence interval.
Top cancer sites among women: breast, lung and colorectal
Substantial increases in breast cancer incidence were observed in the 1980s and 1990s for all groups aged 50 years and older, with APC ranging from 1.33 (95% CI 1.13 to 1.52) in those aged 60–69 years to 2.38 (95% CI 1.86 to 2.90) in those aged 50–59 years (Figure 4). From late 1990s to 2015, rates have been stable in the group aged 30–69 years, except for a large decrease in the group aged 50–59 years during 1999–2005 (APC −2.38, 95% CI −3.73 to −1.01). In the group aged 70–79 years, the rates steadily declined from 1992 to 2005 (APC −0.95, 95% CI −1.40 to −0.49), followed by an increasing trend from 2005 to 2015 (APC 0.85, 95% CI 0.29 to 1.41). There has been a large decrease in the incidence rates among women aged 80–89 years (APC −6.60, 95% CI −7.92 to −5.25). Of note, there has been a considerable increase in the incidence of breast cancer among women aged 20–29 years since 1998 (APC 2.92, 95% CI 1.87 to 3.99).
Age-standardized incidence rates of breast cancer in women in Canada (1971–2015). Note: APC = annual percent change, CI = confidence interval.
Increases in lung cancer rates among women were observed for all age groups (except 20–29 yr) beginning in 1971. More recently, trends have decreased (except 70–79 yr), with rates in women aged 30–49 years now similar to those observed in 1971 (30–39 yr: 1.57 per 100 000 in 1971, 2.03 per 100 000 in 2015; 40–49 yr: 11.33 per 100 000 in 1971 and 11.79 per 100 000 in 2015) (Appendix 3). Recent large decreasing trends have been observed among women aged 30–39 years (APC −5.31, 95% CI −6.42 to −4.20), 40–49 years (APC −6.27, 95% CI −7.34 to −5.18) and 89–89 years (APC −3.78, 95% CI −4.92 to −2.63).
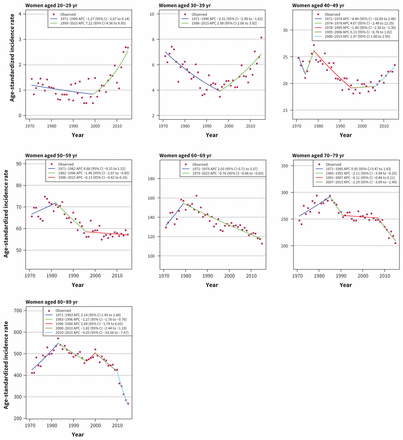
Trends for colorectal cancer among women were similar to those observed among men (Figure 5). Incidence has increased in younger age groups (20–29 yr: APC 7.22, 95% CI 4.56 to 9.95; 30–39 yr: APC 2.98, 95% CI 2.06 to 3.92; 40–49 yr: APC 1.97, 95% CI 1.00 to 2.95) and remained steady or decreased in older age groups (50–59 yr: APC −0.13, 95% CI −0.42 to 0.16; 60–69 yr: APC −0.76, 95% CI −0.86 to −0.65; 70–79 yr: APC −2.29, 95% CI −3.09 to −1.49; 80–89 yr: APC −9.03, 95% CI −10.56 to −7.47).
Age-standardized incidence rates of colorectal cancer in women in Canada (1971–2015). Note: APC = annual percent change, CI = confidence interval.
Cohort effects
Using 1941–1945 as the reference category, we observed that more recent cohorts had numerically increasing IRRs for all cancers combined (among women and men), but trends have not reached significance (Appendix 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1). Among women, significant increased rate ratios were observed for melanoma, and kidney and thyroid cancer, and significant decreases were observed for cervical and ovarian cancer. Numerically but not significantly increasing trends were also seen for breast, pancreatic, esophageal and colorectal cancer (p > 0.05), and decreasing trends for stomach cancers (p > 0.05).
For men, younger cohorts had increased rate ratios compared with the reference cohort for melanoma, prostate, thyroid, anal, kidney, esophageal and liver cancer. Despite these increases, the most recent 1986–1990 cohort appeared to reverse the trend for anal, kidney and liver cancers. The 1986–1990 cohort had a lower incidence compared with the reference cohort for melanoma and esophageal cancer.
Sensitivity analysis
To determine robustness to imputation methods for the final 4 years of Quebec incidence data, we repeated our analyses excluding this province. These age-standardized incidence trends are presented in Appendix 5 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1) and age-standardized APCs excluding Quebec are presented in Appendix 6 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190355/-/DC1). Overall, the results are consistent with the Canada-wide analyses, with the exception of anal cancer among older age groups and thyroid cancer in younger age groups.
Interpretation
Consistent with previous research,1 this extensive analysis of trends in cancer rates in Canada shows that the overall incidence is decreasing among men but increasing among women. The age- and site-standardized trends we observed have several implications for cancer control in Canada. Additionally, results from the birth-cohort analysis provide evidence that may be useful to guide future research.
As the birth-cohort analysis and age-group analysis were based on the same data, they should show trends that are consistent, but the 2 analyses have different purposes. The age-group analysis shows age-specific incidence rates over a period of time. This is useful in identifying changes in practice such as implementation of screening programs, improved diagnosis and changes in coding, as well as a potential shift in risk factors. In contrast, the cohort analysis shows age-standardized IRRs over birth cohorts, which is specific for detecting changes in risk factors. For example, we observed a rise in the lung-cancer incidence rate in the earlier male birth cohorts, with a continuous decrease in the later cohorts, which aligns with the smoking epidemics in the past.
The most striking results from these analyses relate to increasing incidence trends among younger adults for breast, colorectal, pancreatic, endometrial and kidney cancers. Obesity is a risk factor for these cancer sites,12 and the rising incidence runs parallel to the growing prevalence of obesity in recent decades.13 As previously reported, prevalence of class II and III obesity (body mass index ≥ 35) has increased significantly in all age groups,14 but the trend among younger adults is of greater concern because they are ineligible for most cancer screening programs. Relative increases among younger age groups were found for breast cancer, which is a finding consistent with populations in the United States and Europe.15–17 This trend among premenopausal women may reflect a change in risk factors, including later age at first childbirth, increased oral contraceptive use and earlier age at menarche.18–29 A similar trend was noted for colorectal and pancreatic cancer, which has also been seen in the US.30,31 These findings solidify the need for public health measures and interventions that address weight management and are aimed at younger adults. In addition, we observed increases in pancreatic cancer and non-Hodgkin lymphoma among women, a new finding.
Incidence reductions for some cancer sites may be related to successes in both primary and secondary cancer prevention. As reported by others, efforts to reduce smoking initiation and promote cessation have resulted in declines in lung-cancer incidence for men older than 40 years starting in the 1980s.32–34 This analysis shows that lung cancer rates among women are now also declining in all age groups except the 70–79 years category, in which rates continue to climb. Lower smoking rates may have also contributed to a decrease in incidence of bladder cancer in most age groups, particularly those in the younger age categories. 34 Rates of breast cancer among older age groups have declined, which could be related to decreased use of hormone therapy among postmenopausal women.35 The reduction in melanoma among younger men is probably due to increases in sunsafety behaviours in this demographic group. Further work in primary prevention should be aimed at reducing ultraviolet exposure and alcohol consumption, and promoting healthful diets and exercise to address increased rates of liver and kidney cancer, and melanoma.
Secondary prevention efforts have resulted in significant declines of colorectal and cervical cancer. The recent decreases in rates of colorectal cancer among older adults are likely attributable to the use of first endoscopic and late fecal-based screening programs in Canada, which are recommended for adults aged 50–74 years.36 Similarly, the decline in cervical cancer is largely the result of screening to detect treatable lesions37,38 and may be minimally related to vaccination against the human papillomavirus (HPV).39–42 Further decreases in incidence of cervical cancer from organized HPV vaccination can be expected, but this effect is probably not apparent in our results. Vaccination programs for HPV began in Canada in 2006,43 so incidence may continue to decrease as vaccinated cohorts reach ages when cervical cancer is typically diagnosed.44,45 However, the use of prostate-specific antigen testing resulted in sharp increases in the incidence of prostate cancer in 1990 and 2007,46–49 and the overdiagnosis of thyroid cancer has been thoroughly documented.50 New guidelines for prostate cancer screening may be related to decreases in diagnoses in older age groups,48 but the trend of increasing rates of thyroid cancer reflects the need for further research to identify tumour types that are more likely to lead to long-term harms.51
Limitations
This analysis was limited by the available incidence data. Our primary analysis relied on cancer incidence that was imputed for the last 4 years of one of the included provinces (Quebec). In addition, we were able to examine incidence trends only up to 2012 for bladder cancer owing to a recent change in reporting practices in Ontario. Finally, there are subtle differences between provinces in terms of diagnosis and reporting, which could have an influence on our findings. We did not examine any trends in screening, prevalence of risk factors, or other secular trends to examine changes in incidence rates. This could be a point of interest for future research.
Cancer incidence data before 1992 were obtained from the NCIRS, and incidence data after 1992 were obtained from the CCR. The main difference between the 2 databases is that the NCRIS was event-based and the CCR is patient-based.52 Data reported to the NCIRS were reformatted to produce standard records for compatibility with the CCR. All records were reviewed to ensure validity, and less than 2% of records had to be queried by consulting the reporting province.9 Although differences in these databases could be a possible source of error, we wanted to include earlier data to provide additional information on trends. However, any joinpoints around 1992 should be interpreted with caution given the change in data source that year.
Conclusion
Overall, we found large reductions in cancer incidence across age groups for several cancer sites that are probably attributable to efforts in primary and secondary prevention. The overall increase in early-onset cancers, particularly breast and colorectal, highlights the importance of efforts to reduce obesity rates as a measure for cancer prevention. Other successful prevention measures in Canada should continue, and further evidence should be gathered to identify possible emerging risk factors that may be causing cancer in younger cohorts. Future studies should examine similar age-specific incidence trends by province, which may allow the identification of relevant etiologic factors and prevention efforts that are contributing to recent changes.
Footnotes
CMAJ Podcasts: author interview at https://soundcloud.com/cmajpodcasts/190355-res
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Christine Friedenreich and Darren Brenner were responsible for the study conception. Yibing Ruan, Eileen Shaw, Abbey Poirier, Dylan O’Sullivan, Emily Heer, Paul Villeneuve, Stephen Walter, Darren Brenner, Christine Friedenreich, Leah Smith and Prithwish De contributed substantially to the study design and interpretation of the data. Yibing Ruan completed the analysis. Abbey Poirier was responsible for acquisition of the data. Eileen Shaw, Emily Heer, Abbey Poirier and Yibing Ruan were responsible for drafting and revising the manuscript. Dylan O’Sullivan, Paul Villeneuve, Stephen Walter, Darren Brenner, Christine Friedenreich, Leah Smith and Prithwish De critically revised the manuscript for important intellectual content. All of the authors gave final approval of version to be published and agreed to be guarantors of the work.
Funding: Darren Brenner was supported by a Career Development Award in Prevention (no. 703917) from the Canadian Cancer Society. Dylan O’Sullivan is supported by a Queen Elizabeth II Scholarship and an Empire Life Fellowship.
Data sharing: Aggregated data can be accessed on a case-by-case basis; contact the corresponding author.
- Accepted October 23, 2019.













Podcast