Abstract
Background Innovative models of collaborative palliative care are urgently needed to meet gaps in end-of-life care among people with heart failure. We sought to determine whether regionally organized, collaborative, home-based palliative care that involves cardiologists, primary care providers and palliative care specialists, and that uses shared decision-making to promote goal- and need-concordant care for patients with heart failure, was associated with a greater likelihood of patients dying at home than in hospital.
Methods We conducted a population-based matched cohort study of adults who died with chronic heart failure across 2 large health regions in Ontario, Canada, between 2013 and 2019. The primary outcome was location of death. Secondary outcomes included rates of health care use, including unplanned visits to the emergency department, hospital admissions, hospital lengths of stay, admissions to the intensive care unit, number of visits with primary care physicians or cardiologists, number of home visits by palliative care physicians or nurse practitioners, and number of days spent at home.
Results Patients who received regionally organized, collaborative, home-based palliative care (n = 245) had a 48% lower associated risk of dying in hospital (relative risk 52%, 95% confidence interval 44%–66%) compared with the matched cohort (n = 1172) who received usual care, with 101 (41.2%) and 917 (78.2%) patients, respectively, dying in hospital (number needed to treat = 3). Additional associated benefits of the collaborative approach included higher rates of clinician home visits, longer time to first hospital admission, shorter hospital stays and more days spent at home.
Interpretation Adoption of a model of regionally organized, collaborative, home-based palliative care that uses shared decision-making may improve end-of-life outcomes for people with chronic heart failure.
Innovative models of collaborative, interdisciplinary palliative care that use shared decision-making to promote goal- and need-concordant care are urgently needed to meet rising demand among people with heart failure.1,2 Between 2010 and 2015, 75% of people with heart failure in Ontario died in hospital, despite 70% of people preferring an out-of-hospital death and 90% preferring end-of-life health care delivery at home.3–5 Most people also prioritize improvements in quality of life at the end of life over extension of life.6 Admission to hospital near the end of life is often perceived as undesirable and may result in the provision of unwanted care, whereas home visits near the end of life tend to focus on comfort and are associated with higher rates of death at home.4,5,7,8 These preferences are recognized at a system level, such that avoidance of unwanted health care and at-home death are considered quality indicators for end-of-life care.9–11 However, delivering high-quality care for people with heart failure who are near the end of their life is challenging because of their unpredictable illness course and limited capacity of specialist palliative care.4,12–14
Many studies, including a recent meta-analysis, have shown that home-based palliative care is associated with improved quality of life and symptoms, reduced health care use and a higher likelihood of a home death among people with heart failure. 4,8,12,13,15,16 However, only 32% of people with heart failure received home-based palliative care near the end of life in Ontario.4,8 Several randomized controlled trials explored the effects of collaborative care models for people with heart failure; 12–16 the results were mixed regarding quality of life, symptoms and health care use.13,17–21 Some trials reported that palliative care resulted in improvements in quality of life and reductions in burdensome symptoms and hospital admissions, whereas others reported no change in these outcomes.13 Most studies were single centre and none evaluated a model of regional organization and in-person home visits as a scalable approach.
Given the need to address end-of-life care gaps for people with heart failure, we sought to determine whether regionally organized, collaborative, home-based palliative care (CHPC) — involving cardiology, primary care and palliative care — was associated with increased rates of out-of-hospital death among adults who died with heart failure.
Methods
Study design and setting
We conducted a matched cohort study of patients with heart failure who lived within the Toronto Central and the Central Local Health Integration Networks (LHINs) in Ontario, Canada. At the time of the study, these LHINs coordinated public health care services to about 3 million people in urban, suburban and rural municipalities. The LHINs also planned and distributed provincial funding for all public health care regionally. All Ontario residents have access to publicly funded physician and hospital services, and those aged 65 years and older are provided prescription drug insurance coverage. Home care services — provided by physicians, nurses and other allied health professionals — are covered by provincial health insurance.
Data sources
We used patient data from electronic health records at the Temmy Latner Centre for Palliative Care (TLCPC), linked to health administrative databases held at ICES. The TLCPC provides home-based palliative care to about 2000 patients per year across all disease types. We used each study participant’s name, date of birth and unique health insurance number to identify and link their electronic health record data within ICES data sets (Appendix 1, Supplemental Table 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220784/tab-related-content). We then linked all data sets using encoded person-level identifiers. These data sets have previously been used for palliative care research.4,8,12,22–24
Study participants
Our cohort included all adults residing in the Toronto Central and Central LHINs who died with heart failure between April 2013 and December 2019. We identified heart failure using a previously validated method with 84.8% sensitivity and 97.0% specificity. 25 We excluded people who did not reside in the Toronto Central or Central LHINs, those who were not eligible for public health insurance for a continuous period of 3 months or longer during the last year of life, those who did not have a diagnosis of heart failure, those who did not receive any home care services within 2 years before death, those who resided in a nursing home within 1 year before death, those aged younger than 40 years, those who had a left ventricular assist device inserted within 5 years before the index date and those with invalid or missing data. We excluded people who did not receive home care services to ensure all patients in the cohort received the same treatment except for CHPC, as all patients receiving CHPC also receive home care services.
We measured baseline demographic and clinical variables including age, sex, living arrangement (alone, with family or other — a variable captured in the home care database using the resident assessment instrument), neighbourhood income, rurality, recent immigration, comorbidities, percutaneous coronary intervention, cardiovascular device insertion (permanent pacemaker, implantable cardioverter defibrillator) and hospital frailty risk score, using a 5-year or 10-year (for implantable cardioverter defibrillator) look-back period. We identified comorbidities with large economic impact and high prevalence in the general population from the Ontario Health Insurance Plan database and Discharge Abstract Database data using codes from the International Classification of Diseases, 10th Revision, and using validated algorithms for case ascertainment, where available.26 We also measured cardiovascular prescriptions, opioid prescriptions, cardiologist visits, emergency department visits and hospital admissions in the year before the study index date.
Among patients who received CHPC, we measured their treatment preferences at the index date according to documented provider discussions using a methodology described elsewhere. 27 Patients could indicate having more than 1 preference (Appendix 1, Supplemental text). We also measured their New York Heart Association (NYHA) Functional class and their Palliative Performance Score (PPS). We classified their heart failure according to whether their left ventricular ejection fraction (LVEF) was preserved (≥ 50%), mildly reduced (40%–49%) or reduced (< 40%), and by the cause of their heart failure (ischemic or nonischemic). The cause of their heart failure was indicated in their clinical notes and determined by their health care providers, most commonly their cardiologist. These measures were unknown for patients who did not receive CHPC as they are not routinely collected in Ontario’s administrative data.
Exposure
The main exposure was receipt of CHPC under a regional care model that was initially referred to as “Heartfull.” Using the central referral database maintained by the TLCPC, we identified patients who received CHPC with heart failure indicated as the reason for referral, which could be made by any type of provider. The Heartfull regional CHPC model was created in 2013. All patients in the 2 study regions were eligible to receive it and could be referred by cardiologists, outpatient clinics, family physicians and other home-visiting providers. All patients referred for CHPC were assessed at least once in consultation and were captured in the central referral database. After the initial visit, a shared clinical decision was made as to whether the person would benefit from continued follow-up with CHPC. All patients referred for CHPC were included in this study because all received an initial palliative care consultation. The date of first consultation with CHPC (or matched date for unexposed patients) was the study index date.
The CHPC model emphasizes advance care planning and shared decision-making, along with collaboration between specialist palliative care providers, primary care providers and cardiologists. The CHPC model features 3 core components, namely ongoing professional education (e.g., information pamphlets, formal presentations, peer-to-peer support) regarding home-based management of heart failure, standardized protocols for clinical care (including oral and parenteral administration of diuretics)28 and a collaborative interprofessional team of primary care providers, cardiologists and specialist palliative care providers.28–31 The cardiologists remained involved in the patient’s care intermittently until they died and, in the beginning, palliative care physicians frequently called cardiologists to optimize home-based management of heart failure. Care was centrally coordinated through the TLCPC to assign a specialist home palliative care physician and to organize care services.
The initial visit included a consultation with a specialist palliative care physician — often with a nurse, nurse practitioner or home care coordinator — during which they reviewed the patient’s history, identified care needs and a substitute decision-maker, clarified values and preferences, and offered patients and caregivers care supplies and medications for self-management, as well as contact numbers to communicate with the palliative care team 24/7. After the initial visit, the palliative care team contacted the patient’s primary care physician and cardiologist to coordinate activities and update the care plan. The patient’s cardiologist confirmed the nature of the patient’s cardiac disease and prognosis and provided ongoing support for palliative treatment specific to heart failure.
Our comparison group were adults living in the Toronto Central and Central LHINs who did not receive CHPC and who died with heart failure between April 2013 and December 2019. These patients may have received generalist and specialist palliative care at home or in other settings, in addition to ongoing follow-up from their cardiologist and primary care provider in the community.
We classified physicians as palliative care specialists using a previously validated method that showed 76.0% sensitivity and 97.8% specificity.32 We classed physicians who provided palliative care based on their billing codes, but who were not considered specialists, as palliative care generalists.
To minimize the risk of confounding by indication, patients were directly matched on LHIN and presence of dementia at the time of death, and propensity-matched (1:5) on duration of heart failure, date of death and the probability of receiving palliative care, using a propensity score derived from age, sex, living arrangement, presence of chronic conditions at time of death and insertion of an implantable cardioverter defibrillator within 10 years of death. In the propensity model, we used greedy matching with a caliper width equal to 0.2 standard deviation of the logit of the propensity score.
Outcomes
The primary outcome was location of death (out of hospital or in hospital). Hospice deaths were considered out-of-hospital deaths as these occur in a residential-type setting. Deaths in palliative care units were recorded as in hospital deaths, given that these beds are frequently located in a hospital. Secondary outcomes included rates of health care use, including unplanned visits to the emergency department, hospital admissions, hospital lengths of stay, intensive care unit (ICU) admissions, number of visits with primary care providers or cardiologists, the number of home visits with a palliative care physician or nurse practitioner, and the number of days spent at home. We also measured 4 categories of physician-delivered palliative care, derived using different mixes of palliative care fee codes claimed by physicians, based on previous work.4,33
Statistical analysis
We estimated the associations between CHPC, location of death and all secondary outcomes using Poisson generalized estimating equation (GEE) models among the propensity-matched sub-sample (without incorporating the propensity-score into the model) to account for overdispersion, with a robust variance estimator to account for matched sets.34 We modelled all outcomes using Poisson GEE with log person-time of follow-up as the offset parameter to estimate relative risk of occurrence, while incorporating differential follow-up time for each person.
We adjusted modelling for the primary outcome for the presence of renal disease; the number of cardiologist visits; hospital admissions in the year before the index date; cardiovascular devices and procedures; whether the person received home care with an end-of-life designation, which entails dedicated palliative care nursing and case management resources and other services; 16 category of hospital frailty risk score; prescriptions for typical heart failure medications; and the total number of unique medication prescriptions in the year before the index date. We chose covariates to include in the analytical models based on the clinical and research expertise of our team, including those that were imbalanced based on the measured standardized differences after matching. We did not include covariates used for direct matching or those from the propensity score in our analytical models (except for the presence of renal disease and insertion of an implantable cardioverter defibrillator) because these were well balanced after matching (standardized differences < 0.1).34 Secondary outcomes were modelled without adjustment. We assessed balance at index date in our matched cohort using weighted standardized differences.35
We performed 2 predefined sensitivity analyses of the primary outcome. First, we evaluated the outcome among new users of palliative care by excluding patients who received at least 2 palliative care visits in the year before the index date. This new user design minimizes bias by restricting analysis to patients who are starting treatment.36 Second, we excluded medications from the statistical model since prescription records are only available in administrative data for people aged 65 years and older.
We calculated the associated number needed to treat to achieve an out-of-hospital death by bootstrapping randomly selected sets of paired exposed and unexposed patients 1000 times to calculate the estimated crude rate difference and variance in each bootstrap sample, with corresponding 95% confidence intervals (CIs).12
We performed all analyses using SAS version 9.4 (SAS Institute). We considered a 2-sided p value of less than 0.05 to be statistically significant.
Ethics approval
Ethics approval was obtained from Sinai Health’s Research Ethics Board (ID 19–0016-E).
Results
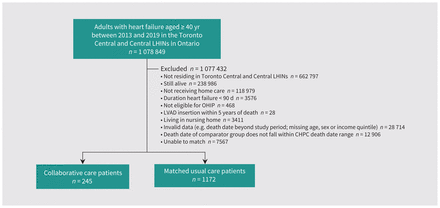
We included a total of 1417 matched patients (n = 245 who received CHPC, n = 1172 who received usual care) (Figure 1). The median duration of follow-up until death for the entire cohort was 81 (interquartile range [IQR] 21–211) days. The mean age was 88.1 (standard deviation 7.9) years, 780 (55.0%) were female and the median duration of heart failure before the index date was 4.5 (IQR 1.5–8.9) years. The most prevalent comorbidities were hypertension (n = 1354, 95.6%), coronary artery disease (n = 991, 69.9%), and primary and metastatic cancer (n = 967, 68.2%) (Table 1). Baseline medications are presented in Table 2. Baseline characteristics of the cohort at time of death are presented in Appendix 1, Supplementary Table 2, and characteristics of unmatched patients are presented in Appendix 1, Supplementary Table 3.
Study flow diagram. Note: CHPC = collaborative home-based palliative care, LHIN = Local Health Integration Network, LVAD = left ventricular assist device, OHIP = Ontario Health Insurance Plan.
Baseline characteristics of patients who died with heart failure, including those who received collaborative home-based palliative care and those who received usual care
Medication use at baseline of patients aged 65 years and older who died with heart failure, including those who received collaborative home-based palliative care and those who received usual care
Among the 245 patients who received CHPC, 41 (16.7%) indicated their willingness to be transferred to hospital for life-prolonging treatment, 77 (31.4%) preferred symptom management but were willing to accept hospital admission, 151 (61.6%) wished to avoid hospital admission and stay home as long as possible, 130 (53.1%) wished exclusively for comfort care and to stay at home and 49 (20.0%) had unknown preferences. Among those with known LVEF, 76 (34.5%) people had reduced LVEF, 18 (8.2%) had heart failure with mildly reduced LVEF and 49 (22.3%) had heart failure with preserved LVEF. Heart failure was due to nonischemic causes in 147 (60.0%) patients. At baseline, 152 (62.0%) patients had a NYHA score of III–IV and 50 (20.4%) had a PPS of 30% or less, indicating a mainly bed-bound status.
Location of death
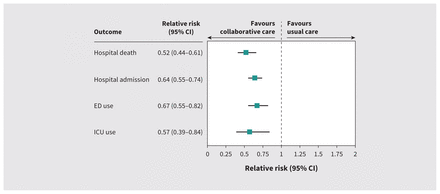
A smaller proportion of patients who received CHPC (n = 101, 41.2%) died in hospital compared with those who received usual care (n = 917, 78.2%). After adjustment, receiving CHPC was significantly associated with a 48% lower risk of dying in hospital compared with usual care (relative risk [RR] 52%, 95% CI 44%–61%) (Figure 2). These results were consistent when including only new users of palliative care (RR 49%, 95% CI 41%–59%) or when excluding medications (RR 51%, 95% CI 43%–61%).
Association between collaborative home-based palliative care, location of death (in hospital v. out of hospital) and health care use (hospital admission, emergency department [ED] use, use of intensive care unit [ICU]) among matched patients who died with heart failure between 2013 and 2019 in the Toronto Central and Central Local Health Integration Networks in Ontario, Canada. Estimates for location of death were adjusted for the presence of renal disease, the number of cardiologist visits, hospital admissions in the year before the index date, cardiovascular devices and procedures, whether the person received home care with an end-of-life designation, hospital frailty risk score category, prescriptions for medications used in the care of people with heart failure (including anticoagulants, β-blockers, mineralocorticoid receptor antagonists, digoxin, furosemide and opioids) and the total number of unique medication prescriptions in the year before the index date. Secondary outcomes of health care use were modelled without adjustment. Note: CI = confidence interval.
Based on these results, CHPC was associated with 1 out-of-hospital death for every 3 (95% CI 3–3) people who received this model of care.
Health care use and clinician visits
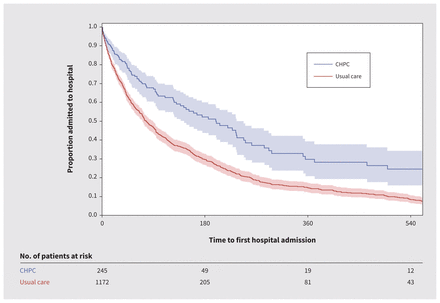
Patients who received CHPC spent more time at home (median 29 d, IQR 13–30 d) than those who received usual care (median 20 d, IQR 9–29 d) (Appendix 1, Supplementary Table 4). Associated health care use was lower among patients who received CHPC, including risk of hospital admission (RR 64%, 95% CI 55%–74%), use of the emergency department (RR 67%, 95% CI 55%–82%) and ICU admission (RR 57%, 95% CI 39%–84%), compared with those who received usual care (Figure 2; Appendix 1, Supplementary Table 4). The time to first hospital admission was longer among patients who received CHPC (hazard ratio 0.39, 95% CI 0.31–0.50) (Figure 3; Appendix 1, Supplementary Table 4), and length of hospital stay was shorter (Appendix 1, Supplementary Table 4).
Kaplan–Meier estimates for time to first hospital admission among patients who received collaborative home-based palliative care (CHPC; blue) and matched patients who received usual care (red), who died with heart failure between 2013 and 2019 in the Toronto Central and Central Local Health Integration Networks in Toronto, Ontario.
Compared with patients who received usual care, patients who received CHPC had higher rates of home visits with palliative physicians (rate ratio 6.3, 95% CI 5.9–6.8), and with nurse practitioners (rate ratio 18.2, 95% CI 15.7–21.0), as well as visits with primary care providers (rate ratio 1.8, 95% CI 1.7–1.8). Patients who received CHPC had lower rates of cardiologist visits (rate ratio 0.9, 95% CI 0.8–1.0) (Appendix 1, Supplementary Table 4).
Categories of physician-delivered palliative care
Palliative care for patients who received CHPC was more often provided using consultative care involving palliative generalists and specialists (n = 114, 46.5%), compared with those who received usual care (n = 88, 7.5%). Patients who received CHPC also received more palliative care from specialist palliative care providers only (n = 127, 51.8%) than those who received usual care (n = 108, 9.2%). Patients who received usual care were provided more palliative care from generalist palliative care providers only (n = 172, 14.7%) than those who received CHPC (n ≤ 6, < 3%).
Processes of collaborative palliative care delivery
Patients who received CHPC were referred from more than 12 different hospitals and community practices. They had a median of 10 (IQR 5–22) in-person visits for palliative care from physicians and nurse practictioners and 5 (IQR 2–11) phone calls from their palliative care team during follow-up. Cardiovascular medications and opioids were adjusted a median of 2 (IQR 1–4) times. Thirty-seven patients (15.1%) died in a dedicated hospice or palliative care unit, where the median length of stay until death was 7 (IQR 2–19) days.
Discussion
We found that regionally organized CHPC — involving multidisciplinary collaboration between cardiology, primary and palliative care providers — was associated with improved health care outcomes in a cohort of patients who died with heart failure, compared with usual care, including an increased likelihood of an out-of-hospital death. Health care systems that adopt and scale such collaborative models through regional organization may improve care alignment and delivery of goal- and need-concordant care.2
The increased need for palliative care is linked to an aging population with rising morbidity and disability,37 and a shortage of palliative specialists to meet growing demands.3,14 Increasing health care capacity through collaborative palliative care models may benefit health care providers, patients and caregivers by enhancing knowledge in caring for complex chronic illness near the end of life.38 This approach may be attractive to policy-makers, as it requires few additional providers; however, it does require reorganization of how care is delivered by an interprofessional team. This approach is supported by recent international guidelines for heart failure.1,39 Scalability does require increased awareness of the model, as well as training and support for the palliative care providers on the team, so they are more comfortable providing appropriate, goal-aligned care to patients with heart failure with consultative support. This model can serve as an exemplar.
Our study aligns with previous work that showed that coordination of health care across regions promoted education and research, and allowed for evaluation of outcomes.40 Similar to our findings, population-based cohort studies showed that palliative care was associated with a twofold increased likelihood of an out-of-hospital death,4,12 and home visits by physicians were associated with a 47% decreased odds of a hospital death.8 A network meta-analysis of randomized trials of patients with heart failure showed that care involving multidisciplinary disease management clinics and home visits with nurses reduced all-cause mortality, compared with usual care.41 Our study contributes to the literature by showing that regionally organized CHPC, compared with usual care, was associated with a lower likelihood of hospital death and decreased likelihood of hospital admission near the end of life.
Limitations
Patients who received CHPC may have been selected by their providers based on underlying treatment preferences of comfort-focused home care, advanced age, higher rates of previous acute health care use, worse severity of illness (reflected by the need for multiple cardiovascular medications) and functional limitations. However, at baseline, nearly half of these patients indicated preferences for active treatment or a willingness to be admitted to hospital (reinforcing that many patients of advanced age and with functional limitations still want active medical interventions). We also randomly selected a control group of matched patients who received usual care, with similar disease status and propensity to be referred to palliative care, who are therefore likely to have unmeasured treatment preferences similar to those who received CHPC. We assumed that patients received CHPC for issues primarily related to their heart failure. However, many patients had multiple comorbidities, which probably contributed to their overall palliative needs and may have influenced their likelihood of requesting palliative care. Relatively few patients were referred to CHPC during the 6 years of eligibility for this study, likely reflecting that this was a new program, and many referring physicians were not aware of it. We did not measure the involvement of potentially important health care providers, such as oncologists, geriatricians and nephrologists. For patients who received usual care, measures such as NYHA class, PPS and LVEF were unavailable in the health administrative data, highlighting the need to improve routine data collection to enable higher quality, patient-centred research. We were unable to assess other important patient-reported outcomes in our administrative data sets, such as a person’s preferences for location of death, their quality of life and their perceptions on quality of death. We assumed that an out-of-hospital death was preferable because the acute care setting is often disruptive, and an out-of-hospital death is preferred by most individuals.5,7 However, some people may not prefer death at home, especially those who have limited supports or resources. Still, an out-of-hospital death is used in several jurisdictions as a system-level quality indicator of end-of-life care.9,42–44 Health care costs rise substantially near the end of life and are largely driven by costs related to hospital admission and acute care,16,24 which we did not measure in this study. Further research is required to determine the cost effectiveness of this model of CHPC for patients with heart failure.
Conclusion
Health care systems should consider adopting and scaling a collaborative home-based model of palliative care, which was associated with improved end-of-life outcomes in patients with heart failure.
Acknowledgements
The authors thank Bhadra Lokuge at the Temmy Latner Centre for Palliative Care for her support of this study and Jahanara Begum for her support in data analysis. The authors thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Footnotes
Competing interests: Leah Steinberg reports participating on the cardiac leadership committee with CorHealth. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and to the acquisition, analysis and interpretation of data. Kieran Quinn and Sarina Isenberg drafted the manuscript, and all of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was supported by a grant from The Global Institute of Psychosocial, Palliative & End-of-Life Care (GIPPEC), the University of Toronto Division of Palliative Medicine and the Dalla Lana School of Public Health, the Bruyère Centre for Individualized Health, the Canadian Institutes for Health Research (PJT-153251) and by a donation from the Grafstein Family to the Sinai Research Foundation.
Data sharing: The data dictionary from the chart review will be available to anyone who wishes to access it upon request, immediately after publication with no end date, and for any purpose. The data set from this study is held securely in coded form at ICES. Although legal data sharing agreements between ICES and data providers (e.g., health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das{at}ices.on.ca). The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer: The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources. Parts of this material are based on data or information compiled and provided by the Canadian Institute for Health Information (CIHI) and the Ontario Ministry of Health (MOH). However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of CIHI or the Ontario MOH. Parts of this report are based on Ontario Registrar General (ORG) information on deaths, the original source of which is ServiceOntario. The views expressed therein are those of the author and do not necessarily reflect those of ORG or the Ministry of Government and Consumer Services. This study was supported by ICES, which is funded by an annual grant from the Ontario MOH and the Ministry of Long-Term Care (MLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH or MLTC is intended or should be inferred.
- Accepted August 29, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/