Hereditary angioedema (HAE) is a rare autosomal dominant disorder characterized by recurrent episodes of painful (and usually asymmetric) swelling without urticaria that leads to substantial morbidity and even mortality (in the case of laryngeal involvement) if left untreated.
Delayed diagnosis and misdiagnosis of HAE are common, particularly during pregnancy and the postpartum period.
Hereditary angioedema should be considered in the differential diagnosis of any patient presenting with unexplained abdominal pain and recurrent episodes of angioedema (particularly if asymmetric in nature) without urticaria.
Tests to confirm the diagnosis of HAE include measurement of C4 and C1 inhibitor (INH) antigen and function.
Successful pregnancy and delivery are possible in HAE with proper medical management, which includes plasma-derived C1-INH and collaboration with HAE specialists.
A 23-year-old pregnant woman (gravida 2, abortion/miscarriage 1, para 0) with a history of anxiety (treated with citalopram) and cocaine use (stopped before pregnancy) and no family history of swelling presented to hospital at 9 weeks gestation. She reported episodic abdominal pain and nausea, as well as vomiting and intermittent unilateral swelling of the hands and feet for the previous 2–4 days. An erythematous nonpruritic rash preceded her symptoms. During the pregnancy, she experienced 4 more episodes with similar symptoms, presenting to either her prenatal care provider or the emergency department. At each presentation, she was assessed for pregnancy complications and reassured that her symptoms would improve postpartum.
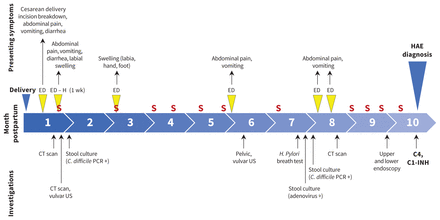
The patient delivered a healthy female infant at term by cesarean delivery under spinal anesthetic and was discharged on postpartum day 2. Eleven days postpartum, the patient presented with swelling and erythema at the cesarean delivery wound. She was diagnosed with cellulitis and managed with intravenous (IV) ceftriaxone, followed by oral antibiotics for 2 weeks with improvement. A wound culture grew Enterobacter cloacae. A few days later, she returned with abdominal pain and pronounced swelling of the left labia and right thigh. A computed tomography (CT) scan of the abdomen and pelvis showed inflammatory changes at the cesarean delivery site with no visible abscess, and nonspecific retroperitoneal inflammatory changes. Her symptoms progressed to include vomiting, diarrhea, abdominal distension and tenderness. She was tachycardic and volume depleted and did not respond to ondansetron and fluid bolus, prompting admission under obstetrics for potential wound infection. Wound examination identified incision breakdown with purulent discharge; wound swab returned polymicrobial flora and was considered nondiagnostic. Clostridioides difficile polymerase chain reaction (PCR) testing of stool was positive, but no toxin was detected. Repeat CT scan showed bowel wall thickening and edema involving the left mid small bowel, with moderate free fluid. Her symptoms were attributed to the wound and C. difficile infection, the infectious disease team recommended ceftriaxone and metronidazole, and she gradually improved over 1 week. She underwent vacuum-assisted closure of the wound and was given ranitidine for possible gastroesophageal reflux.
Over the next 9 months, the patient continued to have symptoms of abdominal pain, nausea, vomiting and episodic, migratory swelling, resulting in frequent presentations to health care providers (Figure 1 and Table 1). In total, she had 6 emergency department presentations and 1 hospital admission and saw 5 different specialists, including obstetrics and gynecology, internal medicine, 2 infectious disease specialists and gastroenterology. She underwent extensive investigations and various diagnoses were suggested (Table 1). Multiple specialists documented her episodes of swelling, but a diagnosis of angioedema was not considered, and no trials of therapies for possible histamine-mediated angioedema (Table 2) were initiated. The patient did not take any angiotensin-converting enzyme (ACE) inhibitor or exogenous estrogen that could have confounded the clinical presentations.
Timeline of swelling episodes and presentations to the emergency department (ED) during the postpartum period of a 23-year-old woman. Note: + = positive, C. difficile = Clostridioides difficile, C1-INH = C1 inhibitor, CT = computed tomography, H (1 wk) = hospital admission of 1-week duration, H. pyrlori = Helicobacter pylori, HAE = hereditary angioedema, PCR = polymerase chain reaction, S = swelling episodes (including vulvar and peripheral [hands, feet, limbs]) documented on assessment or reported by the patient, US = ultrasound.
Postpartum investigations, results, diagnoses and management in a 23-year-old woman
At 10 months postpartum, she was assessed in an outpatient infectious disease clinic, where C4 and C1 inhibitor (C1-INH) levels were ordered. Consistent with a diagnosis of hereditary angioedema (HAE), C1-INH functional assay and C4 were reduced on 2 separate occasions at 10 and 11 months postpartum (C1-INH: 0.18 and 0.37 U/mL, respectively [normal 0.7–1.3 U/mL]; C4: 0.06 and 0.07 g/L, respectively [normal 0.09–0.5 g/L]). A C1-INH antigenic level assay was not performed because the test was not available in the province where she lived. She was referred to Allergy and Clinical Immunology and underwent repeat C1-INH and C4 testing, which remained low. Genetic testing identified a previously unreported missense mutation in exon 8 of SERPING1: c1475T > C, p.(Met492Thr) (classified as a likely pathogenic variant) and we diagnosed type 1 HAE (HAE-1). The patient has responded well to prophylactic C1-INH therapy and icatibant (a selective bradykinin 2 receptor antagonist) as needed for breakthrough swelling.
Discussion
Hereditary angioedema is an autosomal dominant disorder that affects about 1 in 50 000 individuals.3,4 It presents with intermittent episodes of painful, asymmetric swelling without urticaria that affect the extremities and bowel mucosa (resulting in abdominal pain and vomiting) in more than 90% of patients. The face, genitals and upper airways are also frequently affected.5 In about 25% of patients, a nonpruritic erythematous rash (erythema marginatum) precedes the edema.6 Hereditary angioedema is distinct from the more common histamine-mediated angioedema, which is generally associated with urticaria and pruritus, fast onset and offset of symptoms, and response to antihistamines, epinephrine or corticosteroids (Table 2).1,2
Causes and types of HAE
Most cases of HAE are a result of deficiency or dysfunction in C1-INH — a plasma protease inhibitor that mediates the production of the vasodilator bradykinin.3,4 Hereditary angioedema attacks can be triggered by minor trauma, stress or hormonal changes (e.g., pregnancy, menstruation or use of oral contraceptives)3,4 leading to increased levels of bradykinin, which increase vascular permeability and cause angioedema.
Hereditary angioedema is categorized into 3 types (Table 3).3 HAE-1 and −2 are the most common and are caused by mutations in the SERPING1 gene that encodes C1-INH. A third, more uncommon type of HAE (known as HAE with normal C1-INH [HAEnC1-INH]) clinically presents similarly to HAE-1 and −2 but is characterized by normal antigenic and functional levels of C1-INH. HAEnC1-INH is associated with mutations in genes encoding for factor XII, angiopoetin-1, kininogen-1, plasminogen and myoferlin, but many cases are considered idiopathic in the absence of relevant genetic findings.2,3,7,8
Three types of HAE: prevalence and laboratory findings3*
Hereditary angioedema typically presents in the first or second decade of life, with 82% of patients experiencing their first attack before the age of 18 years.9 A familial history is present in most cases; however, about 25% of cases are caused by de novo genetic variants.3
Importance of maintaining a high index of suspicion for HAE
If unrecognized and untreated, HAE leads to substantial morbidity and possibly even mortality if laryngeal edema develops.2,4 Owing to its rarity and features that overlap with other conditions causing swelling or abdominal pain, diagnosis can be challenging and is often delayed.1,4 Abdominal symptoms, which are one of the most commonly reported symptoms in pregnant patients with HAE,10 are generally nonspecific and can mimic various gastrointestinal disorders (e.g., irritable bowel syndrome, gastroenteritis, ulcerative colitis, appendicitis), drug-seeking behaviours11 and pregnancy-related symptoms (e.g., nausea, cramping).1,4
Although our patient had classic symptoms of HAE, several factors likely contributed to the delay in diagnosis, including pregnancy, past substance use, lack of facial edema, lack of family history and age at presentation. Most patients have onset of symptoms in childhood and adolescence, but some present for the first time during pregnancy.4 A retrospective review of 125 full-term pregnancies in women symptomatic with HAE found that 71% had no previous diagnosis of HAE.12 In most, the diagnosis was not made until several years after the pregnancy. Pregnancy can increase, decrease or have no effect on the number or severity of HAE attacks.4
Despite our patient having clear evidence of bowel wall edema on CT scans and impressive, asymmetric swelling in the extremities and labia, the edema was never questioned or documented as angioedema, and no trials of therapy were considered. Our patient is one of the approximately 25% of patients with HAE who do not present with facial swelling during attacks; facial swelling is more easily recognized as angioedema.5
Screening and diagnosis of HAE
Diagnostic laboratory tests for HAE are readily available and inexpensive. They include measuring plasma levels of complement C4 and C1-INH antigen and function (Table 3).3 C1-INH can be temporarily low in pregnancy even in patients without HAE; therefore, testing should be repeated postpartum for confirmation.3,4 Genetic testing should be considered in the absence of family history (as in our case) or if all diagnostic tests are normal (as in the case of HAEnC-INH) and there is still clinical suspicion of HAE.
Management of HAE
Pharmacologic treatment for HAE includes on-demand therapy to reduce the severity and duration of an attack, short-term prophylaxis before exposure to a known or possible trigger, and long-term prophylaxis to reduce the risk of attacks and associated morbidity (see Table 4 for guideline-recommended treatments).3,4
Guideline-recommended treatments for HAE available in Canada that are supported by high-level evidence3
With proper medical management in collaboration with an HAE specialist, successful pregnancy and delivery are possible in people with HAE.4 During pregnancy, delivery and breastfeeding, guidelines recommend plasma-derived C1-INH (a blood product that replaces the deficient C1-INH) for both on-demand and prophylactic treatment, given its long history of efficacy and safety.3 When used as indicated, plasma-derived C1-INH products are well tolerated, with no documented transmission of infectious agents.3 Other treatments in pregnancy (Table 4) could be considered based on shared decision-making with the patient.
Given the risk of an attack, pregnant patients with HAE should deliver in hospital and be closely monitored for at least 72 hours after delivery. Vaginal delivery and epidural anesthesia are preferred because surgery or general anesthesia with endotracheal intubation may trigger swelling.3,4 Plasma-derived C1-INH should be available on the birthing unit for on-demand use if required.
Screening of offspring
Offspring of patients with HAE should be screened. Because C1-INH levels can be falsely low in the first year of life, confirmatory testing should be done after age 1 year.3,4
Resources for patients and health care professionals
A patient organization, HAE Canada (https://haecanada.org/), provides resources for patients and caregivers. The Canadian Hereditary Angioedema Network (https://chaen-rcah.ca/) is a physician organization that also provides resources for patients and health care professionals, including contact information for HAE specialists across Canada.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Acknowledgement
The authors thank Julie Tasso for her talented editorial services and assistance in the preparation of this manuscript.
Footnotes
Competing interests: Victoria Cook reports receiving a consulting fee from Sanofi (outside the current manuscript), and speaking honoraria from Aralez Pharmacuticals and CSL Behring. Dr. Cook also reports holding the following unpaid roles: Continuing Professional Development Vice-Chair for the Canadian Society of Allergy and Clinical Immunology; Chair of Competence Committee for the University of British Columbia Pediatric Allergy and Clinical Immunology Fellowship program; and member of the Canadian Hereditary Angioedema Network, on the Research Committee. Gina Lacuesta reports receiving consulting fees from CSL Behring and Takeda, payment or honoraria from CSL Behring for continuing medical education presentations, and support for travel and accommodations from Takeda to attend the HAE Global Meeting. Dr. Lacuesta holds non-paid positions on the Board of Directors of the Canadian HAE Network, and is a medical advisor for HAE Canada. Dr. Lacuesta has also conducted industry-sponsored research with Takeda. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of this work. Dr. Victoria Cook drafted the initial manuscript, and all authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: CSL Behring Canada Inc. provided an unrestricted grant for manuscript and editorial assistance from a medical editor. The medical editor is not an employee of CSL Behring Canada Inc.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/