Burkholderia pseudomallei is a Gram-negative bacterium found in soil and water of tropical and subtropical countries, with recent locally acquired infections identified in the United States.
Melioidosis, the clinical disease caused by B. pseudomallei, should be considered in a patient returning from an endemic region with fever and pneumonia or abscess of the liver, spleen or prostate, or septic arthritis.
Burkholderia pseudomallei can be difficult to identify by conventional microbiologic techniques.
Microbiology laboratories should be notified if the diagnosis is suspected, to prevent inadvertent exposure for laboratory workers.
A 64-year-old woman presented to the emergency department of a hospital in Toronto, Ontario, with 3 days of left ankle pain and fever. She had a history of diabetes, hypertension and immune thrombocytopenic purpura. Her medications included metformin, sitagliptin, canagliflozin, gliclazide, amlodipine and telmisartan. Six weeks before her presentation, she had returned from Puri, India, where she had been visiting relatives during the winter months. She had walked barefoot in the brackish water of Chilika Lake.
On examination, her temperature was 39.4°C, and her left ankle was swollen, warm and tender. Initial laboratory investigations of note were a normal leukocyte count, hemoglobin level of 123 (normal 137–180) g/L, and an elevated C-reactive protein level of 30 (normal 0–8) mg/L. Blood cultures were negative. A radiograph of her ankle showed diffuse soft-tissue swelling. The emergency physician requested an orthopedic surgery consultation, and joint aspiration was performed, yielding 3 mL of bloody fluid. The Gram stain of the fluid sample showed no organisms and moderate polymorphonuclear leucocytes, and culture was negative. Cefazolin was initiated for empiric management of septic arthritis, selected to cover methicillin-susceptible Staphylococcus aureus.
We admitted the patient for further treatment and observation. She had persistent left ankle pain and fevers. Given her recent travel to India, we performed additional testing. Malaria thick and thin films were negative; serologies for HIV, syphilis, Bartonella, Brucella and Q fever were nonreactive; and stool cultures for bacteria and ova and parasites were negative. Magnetic resonance imaging (MRI) of the patient’s ankle showed a small tibiotalar effusion and subcutaneous edema, for which Orthopedics indicated surgical washout was not required, as this did not suggest septic arthritis.
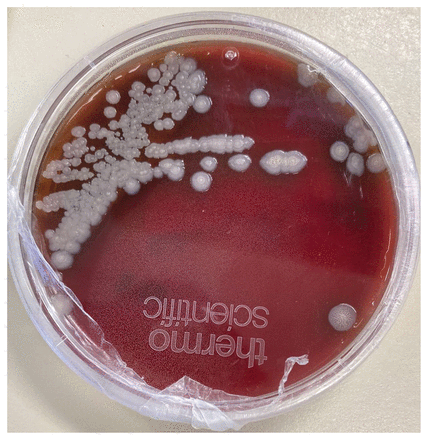
The patient continued to have fever and persistent pain. Interventional radiology performed a second joint aspiration 10 days into her presentation, which yielded 2 mL of pus. No organism was visible on Gram stain, but growth of smooth, grey colonies on blood agar was noted after 24 hours of incubation (Figure 1). We analyzed the colony by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker MALDI Biotyper). The top hit was Burkholderia thailandensis with a score of 1.9, indicating possible species misidentification, wherein scores of 1.7–1.99 can identify organisms to a genus level and scores 2–3 to a species level. Given this result, medical microbiology requested polymerase chain reaction (PCR) testing at both the Public Health Ontario Laboratory and the National Microbiology Laboratory, which identified this isolate as Burkholderia pseudomallei. Antimicrobial susceptibility testing showed that the isolate was susceptible to ceftazidime, imipenem, trimethoprim–sulfamethoxazole, amoxicillin–clavulanic acid and doxycycline.
Growth of Burkholderia pseudomallei on blood agar after 24-hour incubation of fluid aspirated from the ankle of a 64-year-old woman with 3 days of left ankle pain and fevers after travel to India 6 weeks earlier.
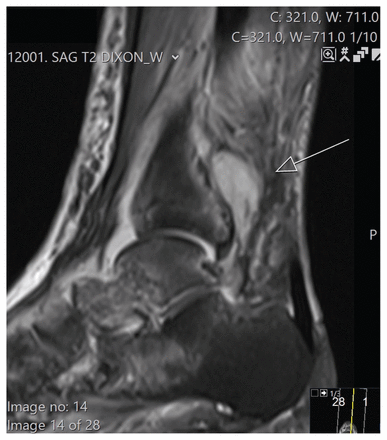
As a result of the laboratory findings, we made the diagnosis of melioidosis. We changed the patient’s antibiotic regimen to trimethoprim–sulfamethoxazole and meropenem, and she defervesced. When the antimicrobial susceptibility testing results were returned, we replaced meropenem with ceftazidime. We obtained a repeat MRI, because the patient had persistent pain. The MRI showed interval progression with findings of septic arthritis, including abscess posterior to the distal tibia, osteomyelitis of the ankle mortise and small subcutaneous abscesses (Figure 2). Chest radiograph did not show evidence of pulmonary disease.
Magnetic resonance imaging of the ankle: sagittal view with T2-weighting showing findings suggestive of abscess with high signal (arrow) posterior to the distal tibia.
The patient underwent arthrotomy, irrigation and débridement of the ankle, 2 weeks into her admission. She received 2 weeks of intravenous meropenem 500 mg every 6 hours, followed by 6 weeks of ceftazidime 2 g every 6 hours, both in combination with oral trimethoprim–sulfamethoxazole, 2 double-strength tablets twice daily. After her course of ceftazidime was complete, she continued oral trimethoprim–sulfamethoxazole monotherapy at the same dose for 2 months. She developed thrombocytopenia (her platelet levels were < 50 on admission, but had ranged from 100 to normal range at baseline) with concern for trimethoprim–sulfamethoxazole adverse reaction and we transitioned her to oral doxycycline 100 mg twice daily to complete a total 6-month course. At her follow-up appointment 4 months after her surgery, she had some residual pain and was walking without assistive devices.
In this case, 3 laboratory workers were inadvertently exposed while performing routine testing on the open bench. Occupational Health deemed their exposures to be low risk, and prophylaxis was not recommended. They were instructed to monitor symptoms and baseline serology for B. pseudomallei was sent to the United States Centers for Disease Control and Prevention, with a plan for convalescent serology in 6 weeks to assess for seroconversion.
Discussion
Epidemiology of Burkholderia pseudomallei
Melioidosis is the clinical disease caused by B. pseudomallei. The organism is found in tropical and subtropical areas, particularly in southeast Asia — with greatest reported incidence in Thailand — as well as northern Australia and the Indian subcontinent. Emerging endemicity has been reported in Brazil and equatorial Africa.1 Locally acquired infections have occurred in the US from exposure to soil in the Gulf Coast states, as well as from imported aromatherapy goods.2 The expanding endemicity is an active area of study and may be related to climate change.1 An estimated 165 000 cases occur globally annually, but underdiagnosis and under-reporting of melioidosis are a major issue.3
The bacteria are ubiquitous in water and soil in endemic areas, and transmission is most common during heavy rain seasons. 3 Burkholderia pseudomallei can survive for many years in moist environments. Transmission occurs via cutaneous inoculation of skin abrasions, inhalation or ingestion. Person-to-person transmission is unlikely; thus, no isolation precautions are required for infected patients. To prevent exposure in endemic settings, water can be boiled before drinking and shoes should be worn by agricultural workers or visitors if direct contact with soil or water is necessary.3
Of adults who develop clinical disease, 80% have medical comorbidities including diabetes mellitus, chronic liver and kidney disease, alcohol use and immunosuppressing medications or diseases.4,5
Clinical presentation of melioidosis and differential diagnosis of septic arthritis
The mean incubation period of B. pseudomallei is 9 days. Like tuberculosis, B. pseudomallei has been called a “great mimicker,” owing to its myriad manifestations and potential to cause symptomatic infection after months to years of latency.4 Mortality is 40% in some endemic regions with limited access to early intensive care. Septic shock occurs in 20% of cases.3,5
Melioidosis presents with a febrile syndrome. The most common manifestation is acute pneumonia, which can progress to lung abscesses and empyema. The infection can disseminate to cause abscesses in the liver, spleen, kidney or prostate. Dissemination to bone and joints, causing osteomyelitis and septic arthritis, occurs in 10% of cases.3 Parotid abscess is a common manifestation in children. Rare central nervous system manifestations include brain abscess and encephalomyelitis. Local cutaneous infection can cause ulceration or subcutaneous abscess.3
Septic arthritis presents with acute monoarthritis.6 Diagnosis is made by joint aspirate fluid leucocytosis typically greater than 50 000/mm3 and growth on bacterial culture. Empiric therapy is guided by Gram stain.6 Staphylococcus aureus is the most common cause globally of septic arthritis in 35%–65% of cases. Other considerations are streptococci, gonococcal arthritis and, less frequently, Gram-negative bacilli.7 A travel history should routinely be obtained on assessment of patients with fever and septic arthritis to consider infections that may not be present locally (Table 1).
Diagnosis and microbiologic considerations
Burkholderia pseudomallei is an aerobic, non-spore-forming Gram-negative bacillus.8 These bacteria grow well on agar plates routinely used for bacterial culture of tissue or fluid specimens. Nevertheless, the differentiation of B. pseudomallei from other Burkholderia species can be challenging.8 MALDI-TOF mass spectrometry is the primary tool for identification of organisms isolated from clinical specimens in most clinical microbiology laboratories. However, B. pseudomallei is not included in the Bruker MALDI-TOF mass spectrometry commercial database. Our patient’s isolate was sent to reference laboratories that used PCR with multiple targets to definitively identify B. pseudomallei.
For diagnosis, blood cultures should be obtained in addition to culture from any suspected site. Repeat specimens should be obtained to increase yield, as blood culture sensitivity is only 50%.3 Burkholderia pseudomallei is not a colonizing organism, so isolation from any site confers a diagnosis of melioidosis.5 Culture remains the gold standard for diagnosis.5 Serology is not typically used for confirmation of the diagnosis as it does not differentiate acute or past infection.5
Burkholderia pseudomallei is a highly pathogenic Risk Group 3 organism, meaning it poses substantial risk of human disease to personnel in the microbiology laboratory. To prevent exposure, laboratory workers must wear proper personal protective equipment and handle the culture in a Level 2 biosafety cabinet. Inadvertent laboratory exposure can occur via inhalation of infectious aerosols or contact with nonintact skin. Thus, clinicians should notify the microbiology laboratory before sending the clinical specimens when B. pseudomallei is suspected. Prophylaxis for laboratory exposure is recommended based on level of exposure risk and presence of comorbidities including pregnancy, diabetes mellitus and immunocompromising conditions. Trimethoprim–sulfamethoxazole or doxycycline initiated within 24 hours of exposure for 21 days have been used as postexposure prophylaxis, but effectiveness is unclear.9 Disease from B. pseudomallei can be difficult to trace given the potential for latent infection. Burkholderia pseudomallei acute and convalescent serology has been used for monitoring seroconversion after exposure.9
Management
Prompt initiation of intravenous antibiotics and resuscitation in the setting of sepsis are crucial. Burkholderia pseudomallei is intrinsically resistant to penicillins, first-and second-generation cephalosporins and aminoglycosides.5 Most isolates are susceptible to ceftazidime, meropenem, imipenem and amoxicillin–clavulanic acid.
Prolonged therapy is required to reduce the risk of relapse, according to retrospective studies in endemic areas.10 In the initial intensive phase of treatment, ceftazidime or meropenem are recommended for 10–14 days; however, this duration may be prolonged, or trimethoprim–sulfamethoxazole may be added for abscess, bone and joint or central nervous system disease. Additionally, abscess drainage or débridement is crucial for source control. In the subsequent eradication phase, trimethoprim–sulfamethoxazole is recommended for a minimum of 3 months, and duration may be extended to 6 months if the patient has central nervous system disease or osteomyelitis.3 Although inferior, amoxicillin–clavulanic acid or doxycycline may be used in instances where trimethoprim–sulfamethoxazole is contraindicated. 5 Recurrent disease occurs in 5% of patients, which may be related to new infection, inadequate source control or an abbreviated phase of intensive treatment.3,4
Conclusion
Melioidosis is an infection acquired in tropical or subtropical regions (including the southern US) that can present after a long latency from acquisition. Clinicians in Canada should consider this infection in patients with a compatible travel history, febrile syndrome and abscess or joint involvement.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Acknowledgements
The authors are grateful for the care provided to this patient by Dr. Mark Downing. We appreciate the technical expertise of Dr. Julianne Kus (Public Health Ontario Laboratory) and the National Microbiology Lab (Winnipeg, Manitoba) Bioforensics Assay Development and Diagnostics Division.
Footnotes
Competing interests: Greg German reports acting as a Board member of Phage Canada (a nonprofit organization). No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Mara Waters and Ellen Avery drafted the manuscript. Greg German, Sigmund Krajden and Yan Chen revised the manuscript critically for important intellectual content. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/