The clinical spectrum of vitamin B12 deficiency can range from asymptomatic to life-threatening; some patients present with pancytopenia and intramedullary hemolysis causing pseudothrombotic microangiopathy (pseudo-TMA).
Vitamin B12 deficiency–associated pseudo-TMA is commonly misdiagnosed as thrombotic thrombocytopenic purpura (TTP); compared with TTP, it is associated with a higher lactate dehydrogenase level, more severe anemia, lower reticulocyte count, more severely elevated mean corpuscular volume and neutropenia.
Treatment of pancytopenia from vitamin B12 deficiency consists of intramuscular injections of vitamin B12.
A vitamin B12 level should be routinely ordered in patients with suspected TMA.
A 76-year-old man presented to the emergency department of a community hospital with a 6-month history of generalized weakness, fatigue, decreased appetite, 25-lb weight loss and exertional dyspnea. He had no infectious symptoms or bleeding. He did not have any personal or family history of hematologic disorders, and his only notable past medical history was vitiligo. On examination in the emergency department, he had scleral icterus and skin hypopigmentation consistent with vitiligo, and his vital signs were normal. He had no palpable hepatosplenomegaly or lymphadenopathy, and his neurologic examination was unremarkable.
Initial bloodwork showed pancytopenia with a hemoglobin level of 45 (normal 135–175) g/L, mean corpuscular volume of 111 (normal 80–100) fL, leukocyte count of 2.9 (normal 4.0–11.0) × 109/L and platelet count of 68 (normal 150–400) × 109/L. His last complete blood count from 3 years ago was normal. Additional investigations showed unconjugated bilirubinemia, markedly elevated lactate dehydrogenase (LDH), schistocytes on peripheral blood film and an inappropriately normal level of reticulocytes, given the severity of his anemia, as well as findings suggestive of coagulation abnormailities. (Table 1). Renal function was normal. High-sensitivity troponin levels and electrocardiography were unremarkable.
Laboratory results included in the initial work-up and evaluation of a 76-year-old man with pseudo-thrombotic microangiopathy caused by severe vitamin B12 deficiency
The patient was admitted to hospital and was seen the next day by an internal medicine physician. He was transfused with 4 units of packed red blood cells for his anemia. Given the combination of hemolysis, thrombocytopenia and presence of schistocytes, the physicians were concerned that he had a thrombotic microangiopathy (TMA), namely thrombotic thrombocytopenic purpura (TTP). The patient’s PLASMIC score (a diagnostic tool for TTP) was 5, representing intermediate risk of severe ADAMTS13 enzyme deficiency.1 He was started on 60 mg oral prednisone daily (1 mg/kg) and a continuous plasma infusion at 100 mL/hour, and was transferred to a tertiary care hospital for consultation with a hematologist and urgent plasma exchange.
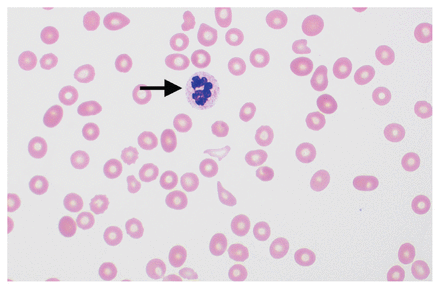
Bloodwork on arrival to the tertiary care hospital showed worsening thrombocytopenia (26 × 109/L), absent haptoglobin and a markedly elevated d-dimer level. The patient’s International Society for Thrombosis and Hemostasis disseminated intravascular coagulation (DIC) score was 7, indicative of overt DIC. We repeated the blood film after transfusion, which was negative for schistocytes but showed hypersegmented neutrophils, thrombocytopenia and red cell anisopoikilocytosis (Figure 1). Additional investigations excluded antiphospholipid syndrome, autoimmune hemolytic anemia and paroxysmal nocturnal hemoglobinuria as a cause of the patient’s condition (Table 1). We obtained a serum vitamin B12 level on day 2 of hospital admission, which was below the laboratory’s limit of detection (< 117 [normal 138–652] pmol/L).
Peripheral blood film of a patient presenting with pseudothrombotic microangiopathy showing a hypersegmented neutrophil (arrow), thrombocytopenia and red cell anisopoikilocytosis (i.e., variation in red cell shape and size), indicating severe vitamin B12 deficiency (Wright-Giemsa stain, ×100 magnification). Image courtesy of Drs. Shreyash Dalmia and Anath Lionel, Department of Medicine, McMaster University, Hamilton, Ont.
Based on the undetectable serum vitamin B12 level, we diagnosed severe vitamin B12 deficiency resulting in pancytopenia, intramedullary hemolysis and DIC. We stopped the plasma infusion and corticosteroids on the third day of admission, and did not start plasma exchange. Several days later, the patient’s ADAMTS13 level was reported to have returned to normal at 56%, with no evidence of anti-ADAMTS13 antibodies, ruling out a diagnosis of TTP.
We administered intramuscular vitamin B12 (1000 μg daily) beginning on the third day of admission, followed by oral vitamin B12 at discharge. The patient’s blood work abnormalities improved over his 7-day hospital stay (Table 2). At a follow-up visit 1 month after discharge, his symptoms had resolved, all hematologic values were improving and his vitamin B12 level were normal. The patient’s anti-parietal cell and anti-intrinsic factor antibodies were negative, which decreased, but did not exclude, the likelihood of pernicious anemia as the cause of his vitamin B12 deficiency.
Laboratory results before and after treatment with intramuscular injections of vitamin B12 for severe vitamin B12 deficiency presenting as pseudo-thrombotic microangiopathy
Discussion
This case illustrates that the clinical spectrum of vitamin B12 deficiency can include life-threatening illness, with hematologic derangements including pancytopenia, pseudo-TMA and overt DIC. Our patient’s severe thrombocytopenia, positive hemolytic markers, schistocytes on peripheral blood film and markedly elevated LDH level (> 1000 U/mL) all supported a possible diagnosis of TMA, with a differential diagnosis including TTP, hemolytic syndrome, atypical hemolytic syndrome or a TMA secondary to drugs, infection or malignant disease. Our differential diagnosis for his elevated LDH included other causes of hemolysis, infection, malignant disease, intracranial hemorrhage, myopathy and multiorgan failure (Box 1). Given his severe vitamin B12 deficiency and normalization of hematologic derangements following vitamin B12 treatment, we diagnosed severe vitamin B12 deficiency. His presentation underscores the importance of ordering a vitamin B12 level for unexplained cases of pancytopenia or suspected TMA.
Box 1: Differential diagnosis for elevated lactate dehydrogenase (> 1000 U/mL)
Hemolysis
Thrombotic microangiopathies such as thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, atypical hemolytic uremic syndrome, disseminated intravascular coagulation, malignant hypertension, scleroderma renal crisis, catastrophic antiphospholipid syndrome
Other causes of intravascular hemolysis such as autoimmune hemolytic anemia, transfusion-related hemolysis, congenital hemolytic anemia (e.g., sickle cell disease), mechanical hemolysis (e.g., burns, prosthetic heart valve dysfunction)
Malignant disease
Lymphoma, leukemia, solid tumours, tumour lysis syndrome
Infections
Pneumocystis pneumonia, tuberculosis, bacterial sepsis, bacterial meningitis, encephalitis, myocarditis, HIV
Multiorgan dysfunction (particularly of lungs, kidneys and liver), often secondary to shock
Myopathy
Rhabdomyolysis, autoimmune myopathies such as polymyositis and dermatomyositis, trauma
Intracranial hemorrhage
Myocardial injury
Type I myocardial infarction, demand ischemia, drug-induced injury
Severe vitamin B12 deficiency
Vitamin B12 is essential for DNA synthesis, production of all hematologic cell lines and myelin synthesis.2,3 The prevalence of vitamin B12 deficiency is estimated to be as high as 6%–20% in the United States.3 Vitamin B12 deficiency is most commonly asymptomatic or mildly symptomatic and is frequently diagnosed in the outpatient setting. Typical symptoms include subacute or chronic onset of fatigue, palpitations, jaundice and neurologic abnormalities, including peripheral neuropathy, loss of proprioception and vibration sense or ataxia. Vitamin B12 is present in foods of animal origin such as red meat, poultry, eggs and dairy products. Common causes of vitamin B12 deficiency include malabsorption secondary to pernicious anemia, gastric or intestinal resection or inflammatory bowel disease, iatrogenic causes such as long-term use of metformin or proton pump inhibitors, or dietary insufficiency secondary to veganism or alcohol use disorder.3,4 The cause of vitamin B12deficiency in our patient was uncertain. Although tests for both anti-parietal cell and anti-intrinsic factor antibodies were negative, we could not rule out pernicious anemia because of the modest test sensitivity of these antibody assays (90% and 60%, respectively).5
Hematologic manifestations of vitamin B12 deficiency include megaloblastic anemia with macrocytic red blood cells and hypersegmented neutrophils, as seen in our patient (Figure 1). More severe deficiency can cause pancytopenia. Elevated methylmalonic acid and homocysteine levels can confirm the diagnosis of vitamin B12 deficiency in cases where the serum vitamin B12 level is inconclusive or borderline low.3,4
Treatment for vitamin B12 deficiency, regardless of cause, is oral or intramuscular vitamin B12 supplementation. The intramuscular route is preferred as initial treatment for patients with neuropathy or pancytopenia.3 Pancytopenia typically resolves within 2 months of starting vitamin supplementation, whereas neurologic deficits may take 3 months or longer to resolve and, in some cases, are irreversible.3,4
In its most severe form, vitamin B12 deficiency can mimic thrombotic microangiopathies such as TTP. Cardinal features overlap with other thrombotic microangiopathies, including anemia with abnormal hemolytic markers, schistocytes on peripheral blood film, elevated LDH and thrombocytopenia (Table 3). It is estimated that 1%–3% of patients with severe vitamin B12 deficiency present with pseudo-TMA.1,6
Bloodwork abnormalities in pseudo-thrombotic microangiopathy (TMA) from vitamin B12 deficiency, compared with thrombotic thrombocytopenic purpura (TTP) and disseminated intravascular coagulation (DIC)
Differentiating pseudo-TMA from TTP is essential, because TTP is associated with 80%–90% mortality if left untreated and is associated with a 1.4-fold increased risk of death and 2.9-fold increased risk of major thrombotic events if treatment is delayed by more than 24 hours.2,7 Acquired TTP is defined by auto-antibodies causing severe ADAMTS13 enzyme deficiency, which is normally responsible for cleaving large, circulating von Willebrand factor multimers. In the absence of ADAMTS13, these multimers accumulate, causing microvascular occlusion (leading to microthrombosis and end-organ injury), platelet consumption (leading to thrombocytopenia) and shearing of red blood cells in the microcirculation (resulting in schistocytes on the peripheral blood film). The PLASMIC score is a clinical screening tool for severe ADAMTS13 deficiency; a high score of 6–7 has a positive predictive value of 72%, whereas a low score of 0–4 virtually rules out a diagnosis of TTP.8 The patient described in this case had a PLASMIC score of 5, neither confirming nor excluding TTP.
A definitive diagnosis of TTP is made by the presence of microangiopathic hemolytic anemia, with an ADAMTS13 level less than 10%. Unfortunately, ADAMTS13 testing has a turn-around time of days to weeks, so immediate empiric treatment for TTP should be started if there is a high index of suspicion. Treatment for TTP consists of immunosuppression and plasma exchange, which removes the ADAMTS13 inhibitor and replaces deficient ADAMTS13 from pooled plasma. Platelet transfusion is generally avoided given a theoretical risk of worsening thrombosis. Transfusion with red blood cells is not contraindicated. In contrast, treatment for pseudo-TMA from vitamin B12 deficiency consists of vitamin supplementation alone.2
Misdiagnosis of severe vitamin B12 deficiency is common in patients with pseudo-TMA. In a 2017 literature review, 39% of pseudo-TMA cases from vitamin B12 deficiency were treated as TTP with plasma infusion or plasma exchange.9 A correct diagnosis was reached after a median of 2 weeks.9 Koshy and colleagues6 compared clinical features of patients with TTP and those with pseudo-TMA secondary to vitamin B12 deficiency between 1997–2019. Compared with patients with TTP, those with pseudo-TMA had more severe anemia (mean hemoglobin 61 v. 94 g/L), higher mean MCV (109 v. 86 fL), higher mean LDH (3539 v. 1183 IU/L), lower mean reticulocyte fraction (0.4% v. 2.4%), lower mean absolute neutrophil count (2.4 v. 7.7 × 109/L) and lower mean total bilirubin (1.6 v. 5.2 mg/dL). In addition, patients with pseudo-TMA typically had normal renal function and intrinsic factor antibodies (69% of patients). The PLASMIC score did not reliably differentiate between these disorders; patients with pseudo-TMA had a median score of 4.9 (intermediate probability of TTP), compared with 6.1 (high probability of TTP) among patients with TTP.
Overt DIC from severe vitamin B12 deficiency, as seen in this patient, is uncommon.10 Disseminated intravascular coagulation is a clinical syndrome caused by widespread and inappropriate activation of coagulation, consumption of coagulation factors, fibrinolysis and production of fibrin degradation products. Typical causes include overwhelming infection, malignant disease, placental abruption and major trauma. Laboratory abnormalities in DIC include elevated d-dimer, severe thrombocytopenia, elevated international normalized ratio and hypofibrinogenemia. Similar to our patient, Aboona and colleagues10 showed that laboratory abnormalities of DIC from vitamin B12 deficiency resolved with vitamin B12 supplementation alone. One proposed mechanism of DIC from vitamin B12 deficiency is apoptosis of immature erythroblasts leading to release of neutrophil extracellular traps, causing microthrombi formation and consumption of coagulation factors.10
Conclusion
We describe a patient who had severe vitamin B12 deficiency causing pancytopenia, pseudo-TMA and DIC. A vitamin B12 level should be routinely ordered in patients with suspected TMA.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/