Ascariasis is a common parasitic infection, particularly in tropical and subtropical regions, with an estimated 800 million to 1.2 billion people infected globally; it should be considered among children newly arrived to Canada.
Although imaging plays a limited role in diagnosis, abdominal ultrasonography may be helpful to evaluate for complications such as bowel obstruction, cholangitis, and biliary or hepatic infiltration.
Treatment is usually with antihelminthics, but children may require endoscopic or surgical intervention for management of complications.
Where possible, children newly arriving to Canada who present with acute medical concerns should be screened for comorbid conditions, using the Caring for Kids New to Canada guideline.
A 5-year-old boy, who had arrived in Canada from a refugee camp in Thailand 3 months earlier, presented to a community hospital with 2 weeks of abdominal pain and decreased oral intake, and 1 day of vomiting white worms.
On examination, the patient was found to have previously undiagnosed physical features of trisomy 21, including low-set ears, flat nasal bridge, macroglossia and bilateral transverse palmar creases. Results from serological testing showed mild transaminitis (alanine transaminase 68 [reference < 50] U/L and aspartate transaminase 86 [reference < 36] U/L), elevated gammaglutamyl transferase (254 [reference 10–22] U/L) and elevated lipase (168 [reference 6–51] U/L). Bilirubin, international normalized ratio, albumin and kidney function were normal.
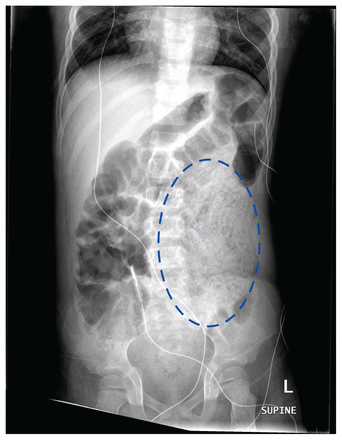
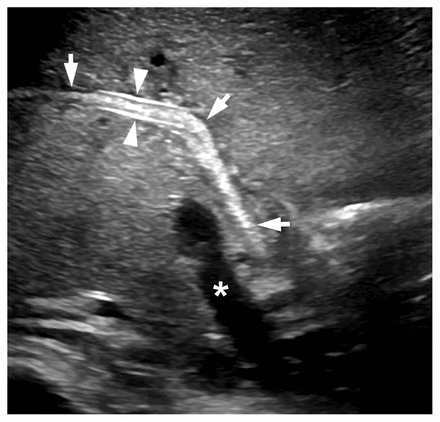
An abdominal radiograph showed an area in the periumbilical region and left flank comprising nodular and curvilinear soft tissue densities (Figure 1). In the context of vomiting worms, these densities were interpreted as a mass of worms within bowel loops. The radiograph did not show evidence of bowel obstruction. Given the patient’s elevated liver enzymes, abdominal ultrasonography was performed, which showed echogenic portal triads, multiple tortuous tubular structures in the gall bladder and dilated biliary tree, consistent with biliary ascariasis (Figure 2). The echogenic portal triads would also have been consistent with infectious cholangitis, but the patient’s clinical presentation did not suggest this diagnosis. No hepatic abscess was seen. A vomited worm, submitted to the laboratory for identification, confirmed infestation with Ascaris spp. (Figure 3). Stool tested for ova and parasites confirmed presence of Ascaris eggs, and no other intestinal parasites were detected.
Supine frontal abdominal radiograph of a 5-year-old boy with biliary ascariasis, obtained at time of patient presentation. A mottled density (dotted oval) of the massed worms filling bowel loops is visible in the left flank.
Abdominal ultrasound of a 5-year-old boy with biliary ascariasis, taken in an oblique sagittal plane through the right hepatic duct and liver porta after start of treatment. A worm is seen in the common and right hepatic ducts (arrows). The parallel walls of the right hepatic duct are prominent (arrowheads) where they are at right angles to the ultrasound beam. *Main portal vein.
An Ascaris lumbricoides worm vomited by a 5-year-old boy.
The patient was transferred to a tertiary pediatric referral centre, and the infectious diseases and gastroenterology teams were consulted. He remained afebrile, appeared well and had no clinical evidence of bowel obstruction. We treated him with oral mebendazole (100 mg by mouth, twice daily). He continued to pass worms per rectum and did not have further emesis during this course of therapy. We considered endoscopic retrograde cholangiopancreatography (ERCP) to remove the Ascaris directly, but medical therapy was preferred owing to the patient’s clinical stability and the technical challenges of performing ERCP in a young child.
After 3 days of treatment with oral mebendazole, repeat abdominal ultrasonography showed reduced burden of Ascaris in the biliary system, although some worms remained. On the presumption that these worms would continue to exit the biliary system and enter the gastrointestinal tract, where they would be amenable to treatment with mebendazole, the course was extended an additional 3 days. After 6 days of treatment, repeat abdominal ultrasonography showed clearance of Ascaris from the biliary system with some residual mild dilatation of the bile ducts. After the extended treatment, the patient continued to pass worms per rectum but in decreasing numbers, with normal stools in between. His transaminase and lipase levels decreased and his oral intake improved.
While in hospital, the patient had a karyotype test, which confirmed a diagnosis of trisomy 21. He had health supervision screening for trisomy 21, which identified primary hypothyroidism, and he was started on levothyroxine. He was also noted to have developmental delay, for which he was referred to local developmental services. He received the remainder of his Caring for Kids New to Canada1 pediatric screening and care. This process identified incomplete immunizations, hearing loss and dental caries that required extractions. Before discharge, the care team arranged follow-up for the patient with a community pediatrician.
One month later, the patient returned for follow-up with the infectious diseases and gastroenterology services. At that time, he was clinically well, and had not had further Ascaris worms in his stool. Repeat abdominal ultrasonography showed continued reduction in bile duct dilatation. The patient was discharged to the care of his community pediatrician. One year later, repeat testing of his transaminase and lipase levels indicated normal liver function.
Discussion
Ascariasis is a parasitic infection of the intestine caused by Ascaris lumbricoides, a helminthic nematode that can grow up to 25–35 cm in length.2 Infection spreads by fecal oral transmission and is common, affecting 800 million to 1.2 billion people globally.3 Prevalence among children in tropical and subtropical regions is about 70%, higher than the 30% prevalence among adults in similar areas.4 Higher rates and more severe disease are associated with lower socioeconomic status, overcrowding and contaminated food and water.2
Most cases of ascariasis are asymptomatic, but an estimated 1–2 million infections per year in endemic areas are clinically symptomatic, ranging from mild abdominal pain to severe manifestations, including bowel obstruction, hepatobiliary involvement and chronic sequelae in children (such as reduced growth, nutritional deficiencies and impaired learning).2,4 Clinical disease occurs most commonly in patients with a heavy worm burden.2,4 Clinicians generally diagnose ascariasis by directly visualizing the relatively large worms passed by patients or by submitting stool samples for microscopic examination for ova.
About 10% of hospital admissions from ascariasis occur because of hepatobiliary involvement, most commonly biliary colic and cholecystitis and, less commonly, infectious cholangitis and hepatic abscesses.5,6 Biliary ascariasis roccurs because Ascaris tends to migrate into bile ducts when there is a high intestinal worm burden;6 it is commonly seen in children in endemic areas4,5 but is uncommon in Canada. Diagnosis is typically made by abdominal ultrasonography, which can suggest the presence of Ascaris in the biliary tree, and by the presence of Ascaris worms or eggs in stool samples.4,7 Characteristic findings on the abdominal ultrasonogram include tubular, linear, echogenic and nonshadowing walls, known as the strip sign; overlapping linear echogenic structures, known as the spaghetti sign; and a single thick, curved or long echogenic nonshadowing stripe with an anechoic centre, known as the inner tube sign.6,7
Children with biliary ascariasis should be evaluated for super-imposed bacterial infection leading to cholangitis or hepatic abscess, related to intestinal bacteria tracking into the biliary tree or liver, along with the Ascaris worms.4–6 Cholangitis typically presents with fever, jaundice, abdominal pain, hepatomegaly, hypotension, elevated conjugated bilirubin and elevated transaminases. Hepatic abscess also typically presents with fever, hepatomegaly and abdominal pain in the right upper quadrant.4 Imaging may be needed to distinguish these 2 diagnoses. Our patient had no signs of associated bacterial infectious complications; however, we had a contingency plan to start piperacillin–tazobactam if the patient developed a fever or otherwise showed signs of bacterial infection.
Oral antihelminthic agents are the mainstay of treatment for biliary ascariasis. These medications paralyze worms in the intestinal tract, allowing for their expulsion by normal peristalsis.4 In our centre, mebendazole was readily available; other options include albendazole, which has not been approved by Health Canada and thus would have been harder for us to obtain, or pyrantel pamoate, which was not a preferred agent.2,6 Mebendazole will not treat worms sequestered in the biliary tree, but once the intestinal worm load is reduced, those that have migrated up the biliary tree typically make their way back to the intestine.6 The typical duration of treatment with mebendazole is 3 days, but can be extended up to 2 weeks. Single-dose therapy with albendazole or pyrantel pamoate is also common; many other variations in medication choice and duration have been reported.4,6–8 As described in our case, children who are clinically stable, without signs of superimposed bacterial infection, can be managed using medical treatment alone, which is effective in about 60%–80% of cases.5 For patients with bowel obstruction, oral antihelminthic agents should not be used until motility is restored. Typically, worms are cleared from the intestine within 3 days, but clearance may take longer depending on intestinal transit time, diarrhea or other host factors.8 Re-examination of stool specimens may be performed 2–3 months after therapy and patients who remain infected can be retreated.9
Endoscopic therapy can be considered if medical treatment is unsuccessful (for example, if worms remain for more than 3 weeks, or if the patient has recurrent biliary colic that does not respond to medical treatment). Surgical consultation is indicated for patients with concurrent bowel obstruction, hepatic abscess or Ascaris in the gallbladder, or for whom endoscopic therapy has failed.4,6
Caring for Kids New to Canada
Immigrant and refugee children and youth new to Canada often have not had a comprehensive health assessment before or after their arrival to Canada. Many factors — including familiarity with the health care system, ethnocultural differences, language discordance and previous traumatic experiences with health care providers — may prevent newly arrived children and families from seeking medical care proactively.10,11 Also, health care may have been limited in the country of origin or refugee setting. As a result, children may have serious health problems that go unnoticed and untreated for months or years before coming to the attention of a medical provider in Canada, as with our patient.
Medical providers for any child new to Canada should not assume that sufficient screening has been done or that families were automatically connected with care at the time of their arrival. The Caring for Kids New to Canada initiative, from the Canadian Paediatric Society, provides detailed guidance for health professionals working with immigrant and refugee children and youth, including checklists of recommended screening questions, physical examination techniques, investigations and follow-up for this patient group, as well as resources to promote culturally safe care (Box 1).1 Comprehensive screening and health care provision for these patients can be time consuming and may require multiple visits. Health care providers should be prepared and supported to take the necessary time to provide this service to a vulnerable and often underserved population.
Box 1: Considerations for the medical evaluation of refugee and immigrant children new to Canada*
History
Chief concern
Present illness
Medical history and past illnesses
Previous medical care
Birth information (including pregnancy and perinatal period)
Medications
Allergies
Immunizations (only accept valid documentation; if reliable documentation is unavailable, assume immunizations not completed)
Dietary history, growth and development
Family medical and social history
Consanguinity
Migration and displacement history
Review of systems
Psychosocial history
Community supports
Adolescent interview
Physical examination
General assessment and vital signs
Growth parameters and nutrition status
Head
Ocular exam
Ear, nose and throat
Dental
Lymph nodes
Cardiovascular
Respiratory
Abdominal
Genitourinary
Pregnancy (in adolescent females)
Musculoskeletal
Neurological
Skin lesions
Investigations†
Complete blood count, ferritin, hemoglobin electrophoresis, glucose-6-phosphate dehydrogenase screen (if indicated)
Urinalysis
Thyroid function
Liver enzymes
Serum creatinine and urea
Serum lead (if indicated)
Tuberculin skin test or interferon gamma release assay (if from an endemic area)
Chest radiography (if indicated)
Stool analysis for culture and ova and parasites (often repeated on 3 separate stools)
Serology for schistosomiasis and strongyloidiasis (if from endemic areas)
Hepatitis A serology, hepatitis B surface antigen and antibody, hepatitis C antibody
Syphilis serology (if indicated)
HIV serology (if indicated)
Malaria smear (if from endemic area and has signs or symptoms [e.g., fever, chills, rigors])
Follow-up visits
Complete history and physical, as outlined above
Follow-up screening labwork and arrange further investigation or referral, as needed
Further developmental assessments
Genetic medical issues
Anticipatory guidance on nutrition, school and injury prevention
Arrange hearing and vision assessment, as needed
Ensure immunizations completed
↵* Adapted from the Caring for Kids New to Canada guidance on medical assessment of refugee and immigrant children.1
↵† Laboratory tests should be ordered based on clinical indications and assessment of risk of various conditions accounting for country of origin, route to Canada and other relevant risk factors, such as family history). Please refer to https://kidsnewtocanada.ca/care/assessment for more details and explanation.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Acknowledgement
The authors acknowledge Dr. Jim. Potts for his contribution in preparing the radiographic and ultrasonound images for this manuscript.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained parental consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/