Patients with suspected cerebral ischemia should be urgently assessed to distinguish between transient ischemic attack (TIA), minor stroke or mimics such as migraine, seizure, vertigo or syncope.
The Canadian TIA Score can be used to determine risk for early subsequent stroke in patients with a suspected TIA or minor stroke.
All patients with TIA or minor stroke should undergo urgent electrocardiography and computed tomography of the head.
Clinicians should order early vascular imaging for patients at moderate or high risk of subsequent stroke; urgent revascularization should be considered if there is more than 50% arterial stenosis congruent with symptom presentation.
Clinicians should prescribe dual antiplatelet drugs for high-risk patients, single antiplatelet agents for low-risk patients or direct oral anticoagulants for patients with atrial fibrillation.
All patients with TIA or minor stroke should be counselled about modifiable lifestyle factors (including smoking cessation), be treated with statins and take steps toward optimizing blood pressure, including treatment with antihypertensive drugs, if necessary.
Suspected transient ischemic attack (TIA) or minor stroke is a relatively common presentation in emergency departments and primary care clinics. Secondary stroke prevention has evolved substantially over the last 20 years, such that the risk of stroke within 90 days of a TIA or minor stroke has fallen from 10% to as low as 1% with optimized and expedited management.1–4
The nomenclature surrounding TIA is inconsistent, which can cause confusion. However, 2 definitions are commonly used. One is based on time (i.e., symptom resolution within 24 hours), and the other is based on the appearance of tissue (i.e., no infarction on magnetic resonance imaging [MRI]).5,6 A recent proposal has argued that it is not important to differentiate TIA from minor stroke, given their common pathophysiology, suggested investigations and management, when thrombolysis or thrombectomy are not indicated.7 Acute ischemic cerebrovascular syndrome has been proposed as a term that includes both TIA and minor stroke.
Nomenclature aside, clinicians must distinguish cerebral ischemic events from stroke mimics (i.e., diagnoses that can resemble TIA or minor stroke), and begin stroke prevention measures in at-risk patients.8,9 About half of initial diagnoses of TIA or minor stroke are ultimately diagnosed as a stroke mimic.10
Once patients are determined to have TIA or minor stroke, a series of investigations and assessments are needed to ascertain the cause as this determines management. Making an accurate diagnosis and identifying high-risk patients in a timely manner is critical to decrease the likelihood of a subsequent event. We discuss the investigation and management of acute ischemic cerebrovascular syndrome based on recent high-quality evidence, position statements and official guidance (Box 1).
Box 1: Evidence used in this review
We reviewed recent position statements for the investigation and management of transient ischemic attack (TIA) or minor stroke. These included the Canadian Stroke Best Practice Recommendations for Secondary Prevention of Stroke Update 2020 and the American Heart Association’s 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack. For articles describing specific mechanisms and treatments, we searched MEDLINE to February 2022, using terms such as “TIA,” “transient ischemic attack” and “stroke.” We primarily considered original articles, but also review articles. We further searched the reference lists of relevant articles to find other articles of interest.
How is transient ischemic attack or minor stroke diagnosed?
The diagnosis of TIA or minor stroke can be challenging and starts with taking a careful and focused history (Figure 1). Classically, a TIA or minor stroke presents with sudden onset of loss of function. Unilateral weakness, aphasia or dysarthria are strongly associated with a high likelihood of TIA or minor stroke. Symptoms are usually not progressive, repetitive, stereotyped or stepwise (e.g., starting in the face, then moving to the arm and then the leg). Although stuttering symptoms, whereby severity fluctuates over a few hours, can occur with small-vessel lacunar strokes, these would not be expected to last beyond 24 hours. Symptoms are usually negative rather than positive; for example, they involve the loss of vision rather than flashing lights, or the loss of feeling rather than electric shocks. Diagnosis is challenging when information is incomplete (e.g., because of language discordance or poor memory) or when patients present with a combination of typical and atypical features.11 Recent studies suggest that clinicians may be less likely to diagnose TIA or minor stroke in women presenting with atypical symptoms than in men, although women have just as high a chance of having cerebral ischemia.12,13 Even low-risk transient events may be associated with infarction on MRI; a recent cohort study found a rate of 13.5%.14 Although some low-risk patients do have cerebral ischemia and would potentially benefit from early imaging, it is not currently clear what impact more widespread MRI scanning would have on health care costs and emergency department crowding or subsequent stroke rates.
Diagnosis and management of transient ischemic attack (TIA) or minor stroke. Note: ASA = acetylsalicylic acid, CT = computed tomography, ECG = electrocardiography.
Common mimics of TIA or minor stroke include migraine, peripheral vertigo, syncope, somatization and seizure.10 Symptoms more commonly seen in patients with a stroke mimic include loss of consciousness, vertigo, bilateral symptoms and confusion.10 Because the resources available to urgently risk-stratify patients for the likelihood of subsequent stroke are limited, physicians should carefully consider other diagnoses before starting such investigations. Some hospitals routinely order an urgent MRI scan given that the longer symptoms are present, the more likely there will be acute ischemic changes on MRI. However, the cost–benefit ratio and the impact of this strategy on subsequent stroke prevention is currently unknown.
How should suspected transient ischemic attack or minor stroke be urgently investigated?
The highest-risk period for a subsequent stroke in patients with TIA or minor stroke is in the first few days after the initial event; the median time is 24 hours.15 Thus, investigations should be done within hours.
Risk stratification
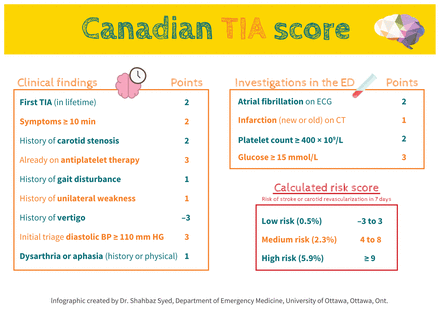
Several scoring systems can be used to identify high-risk patients and guide the prioritization of testing and specialist evaluation. The Canadian TIA Score (Figure 2) has been derived and validated in 2 large, multicentre cohort studies to accurately stratify patients with TIA or minor stroke as low-, medium- or high-risk for a subsequent stroke within 7 days.16,17 Low-risk patients have a risk of less than 1%, medium-risk patients have a 1%–5% risk and high-risk patients have a risk of more than 5% for a subsequent stroke within 7 days. A reasonable clinical approach is to arrange urgent vascular imaging for medium- and high-risk patients, and to discuss high-risk patients with a stroke specialist during their initial evaluation. Most low-risk patients can be managed as outpatients.
The Canadian Transient Ischemic Attack (TIA) score.16 Note: BP = blood pressure, CT = computed tomography, ECG = electrocardiography, ED = emergency department.
The Canadian TIA Score contains 13 clinical or basic investigation variables that are assigned different weights. Free calculators are available through phone applications, such as the Ottawa Rule App, to assist clinicians. The Canadian TIA Score outperformed both the ABCD2 (age, blood pressure, clinical features, duration of symptoms and diabetes) and the ABCD2i (age, blood pressure, clinical features, duration of symptoms, diabetes and infarction) scores, which are relatively simple and widely recognized tools (absolute net reclassification index, which quantifies how well a new model reclassifies cases compared with an old model, of 12.0% and 8.5% over ABCD2 and ABCD2i, respectively; 16.3% of patients classified as low risk with Canadian TIA Score v. 0% classified as low risk using ABCD2 or ABCD2i).16
Neuroimaging
Urgent brain imaging is critical to the assessment of patients with TIA or minor stroke. The Canadian Stroke Best Practice guideline calls for noncontrast computed tomography (CT) of the head to be done immediately for all patients with TIA or minor stroke to assess for stroke mimics and to help determine risk of a subsequent stroke.8 A multicentre cohort study of 1028 patients determined that the patients with abnormalities on CT (an acute cerebral infarction alone, acute plus old infarction, or acute infarction, old infarction and microangiopathy) are at risk for subsequent stroke within 90 days that is 3, 11 and 24 times higher than those with a normal CT, respectively.18 As the sensitivity of CT for TIA or minor stroke is poor, MRI provides additional information but is not immediately available in many Canadian centres.14 Given a relative lack of MRI availability and of clear evidence on the impact of routine MRI, we suggest using the modality that is locally available for neuroimaging, which will be CT in most places.
Vascular imaging
Neck and brain vascular imaging can identify extracranial carotid stenosis, intracranial or posterior circulation stenosis, acute thrombus or cervical artery dissection. It is vital to identify patients with more than 50% stenosis of the extracranial carotid artery with CT angiography or Doppler ultrasonography, as they are at highest risk of subsequent major stroke.19 Given that about half of patients suspected of TIA or stroke actually have a stroke mimic as the cause of their symptoms, it is not a good use of resources to urgently seek imaging for all low-risk patients.10 These patients can either forgo imaging or have imaging performed within days after their index event, depending on the likelihood of the primary event being a true TIA or stroke.
Electrocardiography and Holter monitoring
Electrocardiography (ECG) to screen for atrial fibrillation or flutter should be done immediately for all patients. If the ECG is normal, patients may still be at risk of stroke owing to paroxysmal atrial fibrillation. Subsequent Holter monitoring or loop recording increases the likelihood of identifying atrial fibrillation. Monitoring for up to 14 days in patients with no other cause of their index TIA or stroke identifies more people with atrial fibrillation.20
Echocardiography
Outpatient transthoracic echocardiography can identify cardioembolic causes of TIA or minor stroke other than atrial fibrillation, such as left ventricular akinesis, poor ejection fraction, atrial myxoma, patent foramen ovale (PFO), atrial septal defects, ventricular septal defects and left ventricular thrombus. Because the prevalence of these findings is low, the yield of echocardiography in identifying a treatment-changing cause of stroke is also low. However, positive findings are often important. Because of the low prevalence, no clear recommendations exist to clarify which patients require echocardiography.8 Patients younger than 60 years with a moderate-to-large PFO should be evaluated by a stroke specialist to determine if the PFO is related to the TIA or minor stroke and if it warrants closure.8 Transesophageal echocardiography is generally reserved for patients with an abnormal transthoracic echocardiogram who need detailed assessment of valvular structures or the atrial septal anatomy for planned procedural intervention.
What are the management options for secondary stroke prevention?
Carotid revascularization
Patients with a recent TIA or minor stroke and more than 50% stenosis of the extracranial carotid arteries are at the highest risk of recurrence; the median time to recurrence is 24 hours.15,21 Carotid intervention (carotid endarterectomy or carotid artery stenting) is extremely effective at preventing stroke in these patients; it reduces the absolute risk of subsequent stroke by 17% (95% confidence interval [CI] 11%–24%; with a number needed to treat of 6) in patients with 50%–99% stenosis when treated within 2 weeks of TIA or stroke.9,22 The benefit decreases over time, with no benefit from treatment after 3 months.23 Thus, systems must be in place to identify patients who are candidates for carotid intervention and to intervene quickly.
Antiplatelet agents
The findings of 2 large randomized controlled trials suggest that 21 days of dual antiplatelet therapy (80–81 mg/d acetylsalicylic acid [ASA] and 75 mg/d clopidogrel) is superior to ASA alone for preventing stroke in medium- and high-risk patients.24,25 Dual antiplatelet therapy beyond 21 days is not routinely encouraged as this increases the risk of major bleeding. Single-agent ASA (80–81 mg/d) is appropriate first-line therapy for lower-risk patients, and for most patients after 21 days of dual antiplatelet therapy. When patients have recurrent cerebrovascular events despite antiplatelet therapy, it is important to consider the possibility of alternate causes such as atrial fibrillation, genetic resistance to clopidogrel metabolism or medication nonadherence. When starting antiplatelet therapy, a loading dose should be given (160 mg ASA and either 300 mg or 600 mg clopidogrel). Ticagrelor is an acceptable alternative to clopidogrel for dual antiplatelet therapy with ASA.26,27
Anticoagulation for patients with atrial dysrhythmia
Treatment with anticoagulation results in a 66% relative risk reduction for subsequent stroke among patients with nonrheumatic atrial fibrillation after a TIA or minor stroke.28 In the absence of active bleeding or moderate-to-large acute infarction on CT (i.e., any lesion > 1.5 cm in the anterior or posterior circulation), 29 anticoagulation can be started immediately. A direct oral anticoagulant (DOAC) is recommended over warfarin for nonvalvular atrial fibrillation unless contraindicated (e.g., severe renal impairment [creatinine clearance < 30 mL/min], drug interactions). Follow-up imaging is recommended (usually within 3–7 d) before starting anticoagulation in patients with moderate-to-large infarctions on the initial scan to ensure lack of hemorrhagic transformation. These patients can be started on anticoagulation after 14 days.9
Antihypertensive management
Hypertension is responsible for 39.9% (95% CI 12.5–47.4) of total stroke burden.30,31 Thus, it is one of the most important modifiable risk factors to prevent a subsequent stroke. Optimal targets for systolic and diastolic blood pressure are not known, although a blood pressure lower than 140/90 mm Hg reduces the risk of subsequent stroke.8 If a patient’s symptoms have resolved, no substantial (i.e., > 50%) large artery stenosis is present on vascular imaging, and the patient’s blood pressure is persistently above 140/90 mm Hg, it is reasonable to consider starting antihypertensive drugs immediately.32 If symptoms persist, substantial stenosis is present or no vascular imaging has been conducted, advice from a stroke specialist would be prudent. Angiotensin-converting-enzyme inhibitor with thiazide or a thiazide-like diuretic is recommended, with a calcium-channel blocker as an alternative.8
Statins
All patients with a noncardioembolic TIA or minor stroke, without contraindications and without adverse effects, should be treated with a statin.8 Using a high-dose statin (i.e., atorvastatin 80 mg/d or simvastatin 40 mg/d) is associated with a 1.5%–1.9% absolute risk reduction of subsequent stroke after a median 2.5 years of follow-up.33
Additional measures
It is important to assess all patients for smoking, physical activity, weight and diabetes, and address these risk factors so as to reduce the risk of subsequent stroke. For those with diabetes, a glycated hemoglobin should be assessed, targeting a level below 7.0%.8
Conclusion
Acute cerebral ischemia is a neurologic emergency. A careful history and examination and selected tests can usually differentiate acute cerebrovascular ischemia from stroke mimics. Once diagnosed, it is important to obtain a CT scan and ECG immediately if the event is recent or ongoing. Risk stratification using the Canadian TIA Score will identify low-risk patients who can be managed less urgently, often by their family physician. Medium- and high-risk patients should undergo urgent vascular imaging. High-risk patients should be discussed with a stroke specialist at the time of initial assessment. Patients with carotid stenosis of 50%–99% corresponding to their symptoms, should be referred immediately for surgical consideration. Patients with atrial fibrillation should be started on anticoagulation, usually a DOAC. Clinicians should consider all other patients for a short course of dual antiplatelet therapy, although ASA alone may be appropriate for low-risk patients or those at high-risk of bleeding. Further risk reduction is needed for patients with additional risk factors.
Footnotes
Competing interests: Jeffrey Perry and Michel Shamy report funding from the Heart and Stroke Foundation of Canada and from the Canadian Institutes of Health Research, as well as participation on the Canadian Stroke Best Practice Guidelines group. Michel Shamy also reports funding from the New Frontiers in Research Fund, participation on the data safety monitoring board of the FRONTIERS trial and participation on Canadian Stroke Consortium ethics committee. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/