Abstract
Background: In Canada, low awareness of evidence-based interventions for the clinical management of alcohol use disorder exists among health care providers and people who could benefit from care. To address this gap, the Canadian Research Initiative in Substance Misuse convened a national committee to develop a guideline for the clinical management of high-risk drinking and alcohol use disorder.
Methods: Development of this guideline followed the ADAPTE process, building upon the 2019 British Columbia provincial guideline for alcohol use disorder. A national guideline committee (consisting of 36 members with diverse expertise, including academics, clinicians, people with lived and living experiences of alcohol use, and people who self-identified as Indigenous or Métis) selected priority topics, reviewed evidence and reached consensus on the recommendations. We used the Appraisal of Guidelines for Research and Evaluation Instrument (AGREE II) and the Guidelines International Network’s Principles for Disclosure of Interests and Management of Conflicts to ensure the guideline met international standards for transparency, high quality and methodological rigour. We rated the final recommendations using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool; the recommendations underwent external review by 13 national and international experts and stakeholders.
Recommendations: The guideline includes 15 recommendations that cover screening, diagnosis, withdrawal management and ongoing treatment, including psychosocial treatment interventions, pharmacotherapies and community-based programs. The guideline committee identified a need to emphasize both underused interventions that may be beneficial and common prescribing and other practice patterns that are not evidence based and that may potentially worsen alcohol use outcomes.
Interpretation: The guideline is intended to be a resource for physicians, policymakers and other clinical and nonclinical personnel, as well as individuals, families and communities affected by alcohol use. The recommendations seek to provide a framework for addressing a large burden of unmet treatment and care needs for alcohol use disorder within Canada in an evidence-based manner.
Data from the 2021 Canadian Community Health Survey indicate that about 18% of people aged 15 years or older in Canada meet the clinical criteria for an alcohol use disorder (AUD) in their lifetime. 1 Over 50% of people in Canada aged 15 years or older currently drink more than the amount recommended in Canada’s Guidance on Alcohol and Health, issued in 2023.2
Alcohol consumption in Canada is markedly higher than the global average and above the median for high-income countries. 3 In 2016, more than 4% of all deaths were attributed to alcohol use; alcohol use also contributed to more than 6% of all potential years of life lost for individuals aged 15 years and older in Canada.4 Additionally, alcohol use and AUD represent major contributions to ill health in Canada, with research suggesting that more than 200 health consequences, including injuries and fatalities, are attributable to alcohol use.5
However, evidence-informed interventions for AUD have not been widely implemented in Canada,6 likely owing to structural problems such as stigma and lack of health care provider training, 7 compounded by a lack of national evidence-based guidelines. Although national statistics are lacking, studies from Canadian provinces have shown that less than 2% of eligible patients receive evidence-based alcohol pharmacotherapies.6,8 Additionally, high-risk drinking and AUD are often unrecognized and underdiagnosed in the health care system.9 As a result, people with alcohol problems who seek care are often seen by providers (or services) lacking training in substance use disorders or access to evidence-based resources, which results in ineffective and potentially harmful interventions.10
We developed this national treatment guideline to be a resource for physicians, policy-makers and other clinical and nonclinical personnel, as well as individuals affected by alcohol use. The major aim is to promote the use of evidence-based interventions to reduce alcohol-related harms. A national guideline committee with a broad range of expertise — including clinicians, researchers, people with lived and living experience of alcohol use, and people who self-identified as Indigenous or Métis — was assembled to select priority topics, review the evidence and develop the recommendations.
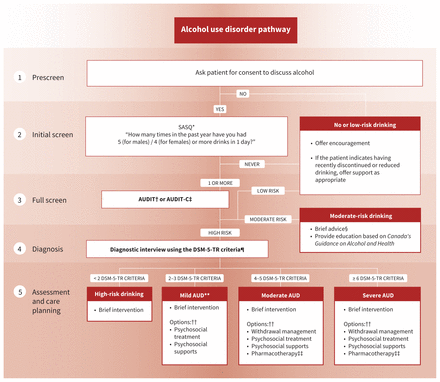
The full guideline is available in Appendix 1 (at www.cmaj.ca/lookup/doi/10.1503/cmaj.230715/tab-related-content) and includes comprehensive evidence summaries for each recommendation, along with practice tools and implementation tips. A visual summary of the guideline is available in Figure 1.
Summary of clinical pathway for alcohol use disorder. DSM-5-TR = Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision.
Scope
This guideline provides direction on the identification and clinical management of high-risk drinking and AUD (see Box 1 for definitions) in adults (aged ≥ 26 yr) and youth (aged 11–25 yr). Brief guidance is also provided specifically for pregnant people, older adults and Indigenous populations. The intended audience is health care professionals in primary care and community-based settings (e.g., rapid access addiction clinics), although some recommendations are relevant to acute care, including withdrawal management programs and emergency departments. The guideline is also intended to be a resource for policy-makers developing health system interventions and for people with alcohol use problems, their families and other affected populations seeking direction on evidence-based care.
Box 1: Definition of alcohol use disorder and risk levels used in this guideline*
Low risk for AUD
Indicated by an AUDIT score of 0–7 or AUDIT-C score of 0–4.
Moderate risk for AUD
Indicated by an AUDIT score of 8–15 or an AUDIT-C score of 5–7.
High risk for AUD
Indicated by an AUDIT score ≥ 16 or AUDIT-C score ≥ 8.
Alcohol use disorder
Alcohol use disorder, as defined by the DSM-5-TR, is diagnosed based on patients meeting the threshold criteria of “clinically significant impairment or distress” from their alcohol use and, among those who meet this threshold, the assessment of 11 diagnostic criteria (see Table 1 for sample interview questions related to DSM-5-TR criteria for diagnosis of AUD).11
The severity of AUD may be mild (2–3 diagnostic criteria met), moderate (4–5 diagnostic criteria met) or severe (6 or more diagnostic criteria met).
↵Note: AUD = alcohol use disorder, AUDIT = Alcohol Use Disorders Identification Test, AUDIT-C = Alcohol Use Disorders Identification Test–Consumption, DSM-5-TR = Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision.
* The AUDIT and AUDIT-C tools were designed to screen for hazardous or harmful alcohol use, using a cut-off score of 8 (for AUDIT) or 3 and 4 for women and men, respectively (for AUDIT-C). In this guideline, we have stratified the scores into low-, moderate- and high-risk categories to facilitate clinical decision-making, based on the guidance in the AUDIT manual.12 Using different cut-off scores will affect the sensitivity and specificity of the tool, as well as false negative and false positive rates.13 The AUDIT-C14 contains the 3 AUDIT questions related to consumption. Both tools have been validated in multiple settings, including primary care.
Sample interview questions for DSM-5-TR criteria for diagnosis of alcohol use disorder
Recommendations
The guideline highlights several overarching principles of care (Box 2) that apply to all recommendations to establish positive partnerships with patients and families experiencing alcohol-related harms. These principles include the importance of considering the social determinants of health and incorporating harm reduction–, trauma- and violence-informed practice and culturally safe approaches as the standard of care for patients and families affected by alcohol use, high-risk drinking and AUD. The guideline highlights the importance of integrating traditional healing and cultural approaches to care in care planning for Indigenous people.
Box 2: Principles of care
Social determinants of health: Alcohol use, high-risk drinking and AUD should be viewed within a larger societal framework that is shaped by inequities in the social determinants of health.65–67 Where appropriate, clinicians should aim to address disparities in the socioeconomic determinants of health by connecting patients with resources that meet these needs (e.g., housing, food and nutrition, financial assistance, employment).
Patient-centred care: Clinicians should strive to provide care that is respectful of the unique needs, values and preferences of each patient.68,69 Patients should be empowered as experts in their own care.
Trauma- and violence-informed practice: Clinicians should be familiar with and incorporate the principles of trauma- and violence-informed practice into the care and clinical management of patients with AUD, with the goal of creating a safe and respectful environment that minimizes the potential for harm and retraumatization.70
Antiracist practices: Confronting and interrogating racist structures in health care and building awareness of one’s own position within oppressive systems can help improve care engagement and health outcomes for racialized populations.71
Indigenous cultural safety and humility: Clinicians should make a meaningful commitment to providing culturally safe care and practising cultural humility in order to establish safe, positive partnerships with Indigenous patients and families.72
Harm reduction: A harm-reduction approach to alcohol use supports any steps taken by patients to improve their health and well-being.73 Clinicians should respect patients’ decisions and goals regarding alcohol use and promote strategies to minimize alcohol-related harms.
Recovery and wellness-oriented care: Clinicians should acknowledge and validate patients’ goals in AUD treatment and care, which may include recovery or self-defined wellness.74
Integrated continuum of care: Alcohol use disorder is understood to be a potentially chronic, relapsing and remitting condition. This guideline supports the use of a stepped and integrated approach, in which treatment options are continually adjusted to meet changing patient needs, circumstances and goals.
Comprehensive health management: Alcohol use disorder should be managed within a broader framework of comprehensive health care and support, including routine and ongoing medical, mental health and psychosocial assessments.75
Family and social circle involvement in care:* Family and social circle involvement in treatment planning and decision-making should be encouraged whenever possible, and when deemed appropriate by the patient and their care team.76–79
Note: AUD = alcohol use disorder.
↵* This guideline uses the term “family” to encompass all relations that are important to the patient within their social circle, which may include romantic partners, close friends and other people of importance who may or may not be legally recognized as family.
This guideline includes 15 recommendations that cover screening, diagnosis, withdrawal management and ongoing treatment, including psychosocial treatment interventions, pharmacotherapies and community-based programs (Table 2). The guideline committee identified a need to emphasize underused interventions that may be beneficial, as well as on common practice patterns that are not evidence based and potentially harmful.
Summary of recommendations
We rated recommendations for certainty of evidence and strength using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (Box 3). In this synopsis, we briefly review recommendations of most relevance to primary care clinicians.
Box 3: GRADE approach and interpretation of grading
The GRADE approach15 assigns a rating for certainty of evidence and strength for each recommendation.
Certainty of evidence
Initial estimates of certainty are based on a traditional hierarchy of evidence, whereby meta-analyses of RCTs are assigned the highest score, followed by individual clinical trials, quasi- or non-randomized trials, observational studies and reports, and expert opinion, which is assigned the lowest score. Factors that lowered confidence in the estimated effect of an intervention included risk of bias, inconsistency across the RCTs, indirectness and publication bias; factors that increased confidence included large effect sizes and an observed dose–response effect. The final certainty ratings are reflective of the estimated effect of an intervention, as reported in the literature, with consideration of biases and limitations of the evidence base as identified by the guideline committee, as described below:
High: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: Any estimate of effect is very uncertain.
Strength of recommendation
To determine strength of recommendations, the GRADE system takes into account the quality of evidence and additional factors, such as clinician, patient and policy-maker’s values and preferences, costs and cost-effectiveness, risk-benefit ratios and feasibility.
A strong recommendation indicates the following:
For patients: Most patients in the given situation would want the recommended course of action and only a small proportion of patients would not.
For clinicians: Most individuals should receive the recommended course of action. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences.
For policy-makers: The recommendation can be adapted as policy in most situations, including for use as performance indicators.
A conditional recommendation indicates the following:
For patients: Most patients in the given situation would want the recommended course of action, but many would not.
For clinicians: Clinicians should recognize that different choices will be appropriate for different patients, and that they must help each patient arrive at a management decision consistent with the patient’s values and preferences. Decision aids may well be useful to help individuals make decisions consistent with their values and preferences. Clinicians should expect to spend more time with patients when working toward a decision.
For policy-makers: Policy-making will require substantial debates and involvement of many stakeholders. Policies are also more likely to vary between regions. Performance indicators would have to acknowledge that adequate deliberation about the management options has taken place.
Note: GRADE = Grading of Recommendations Assessment, Development and Evaluation, RCT = randomized controlled trial.
Screening
Recommendation 2: All adult and youth patients should be screened routinely for alcohol use above low risk (strong recommendation, moderate-certainty evidence).
Implementation of routine and universal alcohol use screening in primary care practice is an important public health strategy for early identification of high-risk alcohol use and secondary prevention of AUD. Although there is insufficient evidence to recommend an optimal frequency for screening,22 the relevant guideline working group suggested that annual screening is practical and feasible in primary care. This recommendation is also relevant to acute care settings where those who may benefit from screening and intervention commonly present.
For adults, we suggest using the Single Alcohol Screening Question (SASQ), which is brief, does not require scoring and is validated in primary care: “How many times in the past year have you had 5 or more drinks in a day (for males) or 4 or more drinks in a day (for females)?”17 This has a sensitivity range of 0.71–0.92 and specificity range of 0.60–0.91 for detecting AUD.17 Positive screens can be followed by the Alcohol Use Disorders Identification Test (AUDIT) or its condensed version, AUDIT–Consumption (AUDIT-C), which have both been validated in primary care and allow for identification of low-, moderate- and high-risk levels of alcohol consumption.12–14,17 When a cut-point of 4 is used on the AUDIT-C to identify either at-risk drinking, hazardous drinking or AUD, the sensitivity ranges from 0.76 to 0.99 and specificity ranges from 0.66 to 0.98.14 For youth, we suggest use of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) screener, with a sensitivity of 0.96 and specificity of 0.85 for AUD when using a threshold of 2 or more drinking days per year.23
We rated the certainty of evidence for this recommendation as moderate, based on systematic reviews and diagnostic accuracy studies that demonstrate that screening tools accurately identify individuals who consume alcohol above low risk levels. 22,24 There are no studies examining the direct impacts of screening on alcohol consumption or health outcomes.22 We rated the strength of this recommendation as strong, given certainty of evidence, guideline committee consensus, cost-effectiveness and accuracy of available screening tools.22 There are no reported harms from screening.22 See Appendix 1, Section 3.2 for additional details on screening and supporting evidence.
Diagnosis
Recommendation 3: All adult and youth patients who screen positive for high-risk alcohol use should undergo a diagnostic interview for AUD using the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision (DSM-5-TR)11 criteria and further assessment to inform a treatment plan, if indicated (strong recommendation, low-certainty evidence).
Adults and youth who screen positive for high-risk drinking should undergo a diagnostic interview for mild, moderate or severe AUD using the DSM-5-TR criteria11 (sample interview questions in Table 1), followed by a conversation about care and treatment goals (Figure 2). This recommendation is relevant to both acute and community settings.
Alcohol use disorder (AUD) care pathway. Note: AUDIT = Alcohol Use Disorders Identification Test, AUDIT-C = AUDIT–Consumption, DSM-5-TR = Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision, SASQ = Single Alcohol Screening Question. *See Appendix 1, Section A2.2. †See Appendix 1, Box 10. ‡See Appendix 1, Box 11. §Brief advice consists of clinician-led feedback on the effects of alcohol, benefits to reducing, and strategies to reduce drinking.2 ¶See Appendix 1, Box 5. **Previously labelled as “alcohol abuse” in DSM-IV. ††Based on patient’s goals and preferences. ‡‡First-line pharmacotherapies are naltrexone and acamprosate.
The diagnosis of AUD can be made when a patient has a problematic pattern of alcohol use over a year that leads to clinically significant impairment or distress, involving at least 2 of the 11 DSM-5-TR criteria. These criteria are: the patient often takes alcohol in larger amounts or over a longer period than they had intended (1); the patient has a persistent desire or has been unsuccessful in their efforts to cut down or control their use of alcohol (2); the patient spends a great deal of time trying to obtain alcohol, using alcohol or recovering from using it (3); the patient has troublesome craving (desire or urges) to use alcohol (4); the patient’s recurrent use of alcohol results in their being unable to fulfill major obligations in their roles at work, school or home (5); the patient continues to use alcohol despite experiencing ongoing or recurrent problems (social or interpersonal) that are caused or worsened because of their alcohol use (6); the patient has given up or reduced social, occupational or recreational activities important to them because of their alcohol use (7); the patient uses alcohol regularly in situations where it is a hazard to them physically (8); the patient continues to use alcohol even though they know they have a persistent or recurrent problem (physical or psychological) that is likely to have been caused by or worsened by alcohol use (9); the patient has tolerance to alcohol use that is demonstrated either by a need for substantially increased amounts to achieve intoxication or the effect that the patient desires, or a substantially decreased effect with their using the same amount of alcohol (10); and the patient has experienced withdrawal that is demonstrated either by taking alcohol or something similar (e.g., a benzodiazepine) to relieve or avoid withdrawal symptoms, or by having the characteristic withdrawal syndrome (11). Patients are diagnosed with mild AUD if they have 2–3 criteria; moderate AUD with 4–5 criteria; and severe AUD with 6 or more criteria.
Although false-positive diagnoses are possible with the DSM-5-TR AUD criteria, which have been subject to some criticism for merging the separate categories of alcohol “abuse” and alcohol “dependence” from the previous DSM-IV iteration,25 current North American practice involves a diagnosis of AUD severity and a comprehensive patient assessment to determine the treatment pathway and care plan.
We rated the certainty of evidence for this recommendation as low by guideline committee consensus, given lack of research on the impacts of diagnosing mild, moderate or severe AUD. However, we rated the strength of this recommendation as strong, based on guideline committee consensus, the recognized need for diagnosis and grading of severity to enable patients to access further AUD care and available evidence.9 See Appendix 1, Section 3.3 for additional information and supporting evidence on diagnosis.
Brief intervention
Recommendation 4: All patients who screen positive for high-risk alcohol use should be offered brief intervention (strong recommendation, moderate-certainty evidence).
All patients who are identified with high-risk alcohol use from the screening stage should receive a diagnostic interview, followed by brief intervention as the first step in developing a care plan. This includes patients who screen positive for high-risk alcohol use or are diagnosed with an AUD.
Brief intervention that uses the techniques of motivational interviewing can be offered by a variety of health professionals, with demonstrated efficacy after a single 5-minute session.26 A Cochrane review in 2018 found that brief intervention results in a reduction of alcohol consumption of 20 g per week after 1 year, compared with minimal or no intervention (95% confidence interval [CI] −28 g to −12 g).26 Several approaches to brief intervention and population-specific considerations are discussed in Appendix 1, Sections 3.4 and 3.5.
Typically, brief intervention involves a short conversation to discuss the patient’s health concerns, collaboratively set goals and develop a treatment plan tailored to those goals and patient preferences. As discussed below, the treatment approach for AUD may include withdrawal management, psychosocial interventions and pharmacotherapies.
We rated the certainty of evidence for this recommendation as moderate, based on systematic reviews that found brief intervention resulted in significant and clinically meaningful reductions in high-risk drinking behaviours.26,27 We rated the recommendation as strong, based on quality of evidence, guideline committee consensus, cost-effectiveness, the effectiveness of brief intervention and the lack of reported harms.22
Withdrawal management
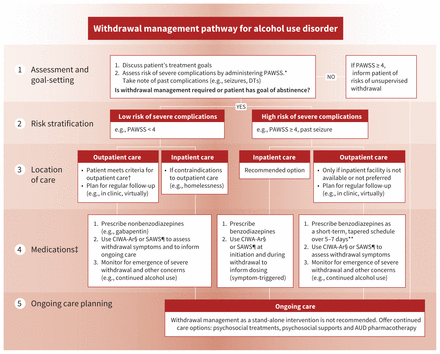
Recommendation 5: Clinicians should use clinical parameters, such as past seizures or past delirium tremens, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) to assess the risk of severe alcohol withdrawal complications and determine an appropriate withdrawal management pathway (strong recommendation, moderate-certainty evidence).
Patients requiring (e.g., admitted to hospital or incarcerated) or seeking abstinence should be offered withdrawal management support, but most people with AUD will not develop complicated withdrawal.28 However, the PAWSS tool28 should be administered to all patients with AUD, even if they decline withdrawal support, to inform them of the risks of abruptly stopping their alcohol consumption. For those identified as being at high risk for severe withdrawal complications, the risks of unsupervised withdrawal can be life threatening.16 Therefore, this recommendation emphasizes the importance of identifying patients who may be at high risk of life-threatening complications, including severe alcohol withdrawal (e.g., seizure, delirium tremens), and employing risk-based stratification to select the most appropriate withdrawal management setting and pharmacotherapies. This recommendation is relevant to both community and acute care settings for withdrawal.
The PAWSS was validated in inpatient settings28 to assess risk, with studies reporting that PAWSS has a very high ability to discern those at high (likelihood ratio 174, 95% CI 43 to 696; specificity 0.93 with ≥ 4 findings on PAWSS) versus low (likelihood ratio 0.07, 95% CI 0.02 to 0.26; sensitivity 0.99 with ≤ 3 findings on PAWSS) risk of developing severe alcohol withdrawal syndrome. 16 We recommend the PAWSS score be used to triage patients to inpatient versus outpatient withdrawal management. However, individuals with a PAWSS score of less than 4 should be considered for inpatient withdrawal management if there are barriers to community withdrawal management, or if there is a history of complicated alcohol withdrawal.16
A detailed overview of withdrawal management strategies, including thiamine supplementation and pharmacotherapies, that are tailored to outpatient (e.g., primary care offices) and inpatient settings is provided in Appendix 1, Section 4, and summarized in Figure 3.
Withdrawal management pathway for alcohol use disorder. Note: AUD = alcohol use disorder, BID = twice daily, CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol–Revised, DTs = delirium tremens, PAWSS = Prediction of Alcohol Withdrawal Severity Scale, QID = 4 times daily, SAWS = Short Alcohol Withdrawal Scale. *See Appendix 1, Box 16. †See Appendix 1, Box 7. ‡Offer oral thiamine (200 mg daily) before and during withdrawal management. In inpatient settings, offer parenteral thiamine (200–300 mg daily) for patients with suspected Wernicke encephalopathy, decompensated liver disease, or at risk of malnourishment, for 5 days minimum, followed by oral thiamine. §See Appendix 1, Box 14. ¶See Appendix 1, Box 15. **Example prescription for severe withdrawal: diazepam 10 mg BID-QID (days 1–3), 5 mg BID-QID (days 4–5), then reassess for days 6–7. Adjust daily based on symptoms and consider daily dispensing or blister packaging.
We rated the certainty of evidence for this recommendation as moderate because the PAWSS has shown strong accuracy in a small number of prospective studies in limited populations.16,28 We rated this recommendation as strong given the certainty of evidence, guideline committee consensus, cost-effectiveness, feasibility of implementing PAWSS in clinical settings and the usefulness of risk stratification to inform patient care pathways.16
Treatment and ongoing care
Psychosocial treatment interventions
Recommendation 9: Adult and youth patients with mild to severe AUD should be offered information about and referrals to specialist-led psychosocial treatment interventions in the community (strong recommendation, moderate-certainty evidence).
Various psychosocial treatment interventions are available and effective for AUD and are reviewed in Appendix 1, Section 5. Research indicates that cognitive behavioural therapy and family-based therapy have small to medium beneficial impacts on AUD outcomes.29,30 For example, when compared with individualized treatment, family-based therapy resulted in increased days abstinent or without heavy substance use at 12-month follow-up (Hedges’ g = 0.47, 95% CI 0.34 to 0.61 [medium effect size]).30 These therapy modalities are delivered by specialists, meaning that appropriate training or education specific to those modalities is required. This recommendation is relevant to both acute and community care settings, as referrals can be beneficial in both instances.
We rated the certainty of evidence for this recommendation as moderate, based on several meta-analyses and randomized controlled trials (RCTs) that have shown that psychosocial treatment interventions result in small to moderate treatment effects on various alcohol outcomes.16,28 We rated this recommendation as strong, given the certainty of evidence, guideline committee consensus, the effectiveness of psychosocial treatment interventions and the benefits of psychosocial treatment interventions relative to the potential risks.
Pharmacotherapy
Recommendation 10: Adult patients with moderate to severe AUD should be offered naltrexone or acamprosate as a first-line pharmacotherapy to support achievement of patient-identified treatment goals (strong recommendation, high-certainty of evidence).
To complement psychosocial treatment, a key recommendation is that adults with moderate to severe AUD should also be offered pharmacotherapy in primary care, with naltrexone or acamprosate as a first-line therapy to support achievement of patient-identified treatment goals and preferences (Table 3). A detailed discussion of setting patient-centred treatment goals is available in Appendix 1, Section 6.1.
Pharmacotherapy for alcohol use disorder*†
Naltrexone is recommended for adults who have a treatment goal of either abstinence or a reduction in alcohol consumption with an estimated number needed to treat to prevent a return to heavy drinking of 12 (95% CI 8 to 26).18 Alternatively, acamprosate is recommended for patients who have a treatment goal of abstinence, with an estimated number needed to treat to prevent return to any drinking of 12 (95% CI 8 to 26).18 Both medications have a well-established, high-quality evidence base for safety and efficacy in AUD for adults.18,19 Naltrexone is contraindicated in people who are on chronic opioid therapy and people with acute hepatitis. Common adverse effects include nausea, dizziness and fatigue, and are usually temporary.18
Treatment for youth often initially consists of psychosocial interventions alone, as most AUD medications have not been studied for safety and efficacy in this age group. However, the guideline committee suggests that naltrexone and acamprosate may be offered to youth for treatment of moderate to severe AUD on a case-by-case basis.
We rated the certainty of evidence for this recommendation as high, based on multiple systematic reviews that indicated naltrexone is effective for reducing alcohol consumption and maintaining abstinence, and acamprosate is effective for maintaining abstinence. 18 We rated this recommendation as strong, given the quality of evidence, guideline committee consensus, cost-effectiveness and the effectiveness of naltrexone and acamprosate.
Other recommended pharmacotherapy strategies, including off-label medications (i.e., gabapentin and topiramate), are summarized in Table 3. The evidence reviews for these and additional pharmacotherapies (e.g., disulfiram) are provided in Appendix 1, Section 6.
In addition to reducing alcohol consumption, pharmacotherapies have shown long-term health benefits. A 2022 retrospective cohort study of more than 9600 patients with AUD over a 9-year follow-up period found that many AUD pharmacotherapies were associated with decreased incidence of alcohol-associated liver disease, and hepatic decompensation in patients with cirrhosis.31 These findings are consistent with a meta-analysis showing the major impact of lower alcohol exposure in reducing mortality risk for those with AUD.32
Research evidence to guide the optimal duration of AUD pharmacotherapy is lacking. The guideline committee suggests a minimum duration of 6 months, at which point the utility of continuing treatment can be reassessed. Although most AUD pharmacotherapy is prescribed in community settings, this recommendation is relevant to acute care settings, such as withdrawal management facilities and other acute care environments where people with AUD may present for care. Effective pharmacotherapies should be paired with evidence-based psychosocial treatments and supports where possible and according to patient goals.
Recommendation 12: Adult and youth patients should not be prescribed antipsychotics or selective serotonin reuptake inhibitor (SSRI) antidepressants for the treatment of AUD (strong recommendation, moderate-certainty evidence).
Recommendation 13: Prescribing SSRI antidepressants is not recommended for adult and youth patients with AUD and a concurrent anxiety or depressive disorder (strong recommendation, moderate-certainty evidence).
Early in the guideline development process, the committee identified that polypharmacy was common among people with AUD and that these patients are routinely offered pharmacotherapies that may be ineffective and potentially worsen AUD outcomes.10 For instance, modern antidepressants (including SSRIs and trazodone), as well as antipsychotics (e.g., quetiapine), are widely prescribed to people with AUD — often to treat symptoms attributable to AUD.10 However, meta-analyses of RCTs have concluded that these medications have little benefit in AUD (e.g., no improvement in depression symptoms with SSRI in those with AUD), even when there are on-label indications for their use.33,34
Importantly, a case series35 and several RCTs36,37 have demonstrated that certain serotonergic medications may worsen AUD outcomes in some patients. For example, in a large Canadian RCT of individuals with AUD (n = 265), 60% of whom had concurrent depression, SSRI treatment was not more effective than placebo in reducing depression symptoms at 12-week follow-up.36 Concerningly, SSRI treatment was associated with a higher number of heavy drinking days and poorer outcomes on other measures of alcohol consumption than placebo.36 Similarly, a double-blind RCT of trazodone (an antidepressant often used off label for alcohol-associated insomnia) showed that, in comparison with placebo, the trazodone group used more alcohol both while on trazodone and when the medication was stopped.37 These observations regarding possible increases in alcohol use in certain individuals when prescribed serotonergic medications may have underlying genetic explanations38 and are consistent with a range of nonhuman laboratory experiments39 as well as randomized trials in other substance use disorders.40,41
Because of the potential increase in alcohol consumption, lack of benefit in meta-analyses of randomized trials34 and other risks (e.g., lowering seizure threshold),42 we recommend against the use of SSRI antidepressants for the treatment of AUD, or for anxiety or depressive disorders concurrent with AUD. These recommendations apply primarily to outpatient primary care environments, where most antidepressant medications are prescribed.
Similar observations have been made in some RCTs when antipsychotics are prescribed for AUD,43 and because of a lack of benefit in meta-analyses,33 we caution against antipsychotic use in AUD. For instance, in a 6-month double-blind placebo-controlled RCT examining the antipsychotic flupenthixol among more than 180 people with AUD, individuals randomized to the antipsychotic experienced a higher rate of relapse than with placebo.44 Although food craving and potential for increased tobacco use has long been known to be a consequence of antipsychotic use, emerging evidence suggests that chronic antipsychotic exposure may enhance motivation for other drug use in some patients.45–47
We rated the certainty of the evidence for these 2 recommendations as moderate, based on 2 systematic reviews and several RCTs studying use of SSRI antidepressants and antipsychotics among people with AUD and without concurrent mental health disorders.33,34 We rated these recommendations as strong, given the certainty of the evidence base, guideline committee consensus and known possible costs and harms. For more information on the evidence supporting our recommendations, see Appendix 1, Section 6.10.
Although these recommendations should apply to most patients with AUD, in patients with a history of a diagnosed mental health disorder and demonstrated benefit from SSRI therapy, continued use of the medication could be considered with close monitoring of clinical response as well as unintended effects (e.g., increased alcohol consumption).
Further study is warranted on the issues related to prescribing of antidepressants, antipsychotics and benzodiazepines in management of AUD, and this guideline does not address severe psychiatric conditions when prescribing these medications (according to on-label indications) may be appropriate (e.g., antipsychotic use in a person with schizophrenia and AUD). However, given available meta-analyses,33,34 we recommend against common prescribing practices that often result in polypharmacy and may be of little benefit in most patients with AUD and where other interventions may be safer and more effective.
For patients with severe concurrent mental health challenges, we support referral to a specialist with appropriate expertise for assessment and evidence-based treatment. We also stress the need to improve access to evidence-based mental health and addiction care for those with AUD.
Recommendation 14: Benzodiazepines should not be prescribed as ongoing treatment for AUD (strong recommendation, high-certainty evidence).
Because short-course benzodiazepine therapy has been shown to be beneficial in alcohol withdrawal management but may be addictive,48 it has been well described that individuals with AUD may end up on chronic benzodiazepine therapy with resultant harms.49,50 Therefore, in the guideline, we reinforce that benzodiazepine prescribing in people with AUD should be limited to short-course treatment to facilitate alcohol withdrawal management in those at risk of severe withdrawal. This recommendation is targeted to both community and acute care environments. We rated the certainty of evidence for this recommendation as high, based on multiple meta-analyses and RCTs showing the harms related to benzodiazepine use, potential for nonmedical use, and documentation of the serious adverse effects and events, including falls and injuries.49,50 We rated this recommendation as strong, given guideline committee consensus and known possible harms of benzodiazepine use among people with and without AUD.49,50
Methods
The guideline development was overseen by a steering committee based within the Canadian Research Initiative on Substance Misuse (CRISM), a national network composed of 5 regional nodes across Canada. We used the Appraisal of Guidelines for Research and Evaluation Instrument (AGREE II)51 to ensure the guideline met international standards for transparency, high quality and methodological rigour. Guideline development followed the ADAPTE process, 52 building upon a pre-existing British Columbia guideline.53 We used the GRADE tool15 to score recommendations (Box 3).
Guideline development activities were supported by grant funding from Health Canada’s Substance Use and Addictions Program (2021-HQ-000066). The guideline was also supported by Canadian Institutes of Health Research funding to CRISM, and the BC Centre on Substance Use provided in-kind support. The guideline was developed between December 2020 and July 2023.
Composition of participating groups
The steering committee comprised the co-chairs of the Canadian Alcohol Use Disorder Guideline Committee (E.W., J.R.), an associated program director (J.W.G.R.), a guideline development manager (N.G.), a guideline coordinator (A.H.) and 2 medical writers (J.B., K.H.).
The interdisciplinary guideline committee of 36 individuals was assembled via outreach conducted by the CRISM Node Managers and Node Principal Investigators in December 2020. The committee included representation from across Canada, with expertise spanning addiction medicine, family practice, evidence-based medicine, mental health, social work, nursing, pharmacy, recovery-oriented systems of care, health care administration and policy. Additionally, the committee included 4 people who self-identified as Indigenous or Métis and 11 people with lived and living experience of alcohol use. Three working groups were developed to focus on screening, diagnosis and brief intervention; withdrawal management; and treatment and ongoing care.
Selection of priority topics
The overarching principles, outline, scope and contents of the guideline were approved by consensus of the full committee upon discussion of the content of the British Columbia Guideline and integrating suggestions from committee members. A full list of research questions and inclusion and exclusion criteria is available in Appendix 1, Section A1.4.
Literature review and quality assessment
We performed an updated systematic literature search in September 2020 using the same search strategies employed in the BC AUD guideline.53 In brief, a contracted information specialist performed literature searches of the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials via Ovid; and CINAHL and PsycINFO via EBSCOHost. We screened only new results not previously identified for the BC AUD guideline for inclusion. Two staff medical writers (including J.B.) independently screened and identified eligible studies. Discordance between reviewers on inclusion or exclusion of individual studies was resolved through discussion. An experienced reviewer (J.B.) used validated assessment tools (e.g., A Measurement Tool to Assess Systematic Reviews, version 2 [AMSTAR-2], Cochrane Risk of Bias Tool, Downs and Black checklist) to evaluate study quality. 54 We also conducted a targeted search in May 2022 to examine questions related to polypharmacy in AUD.
Development of recommendations
We updated evidence summaries from the BC AUD guideline53 with newly identified literature and shared them with the relevant working groups. Each working group determined through consensus whether the recommendations should be accepted without modification, adapted or removed. Working groups conferred over email and video conference between December 2020 and December 2022.
Once the working groups approved the wording and grading of the recommendations and supporting text for their respective sections, we compiled and circulated them for review to the full committee. Subsequently, a committee meeting was held in December 2022 to discuss the feedback, which we then incorporated into a revised draft. The committee approved the revisions by consensus before external review.
External review
We circulated the draft guideline for review and comment to 13 relevant experts and stakeholders from Canada and international jurisdictions in January 2023. Expertise among external reviewers included addiction medicine, psychiatry, psychology, evidence-based medicine and Indigenous health. All external reviewers completed disclosure of competing interest forms before review. A staff medical writer (K.H.) incorporated feedback from the external reviewers — including clarity on screening tool validation, greater emphasis and description of psychosocial treatment interventions, and clearer presentation of recommendations — into a revised guideline. We then recirculated the final document to the full committee for final review, comment and sign off by consensus. The composition of the external reviewer panel is listed in Appendix 1, Authors and Contributors.
Management of competing interests
In keeping with Guidelines International Network’s Principles for Disclosure of Interests and Management of Conflicts,55 committee members were required to disclose competing interests in November 2020 and May 2023. The guideline’s Competing Interest Adjudicator (N.G., with support from the co-chairs) did not deem any of the potential direct or indirect conflicts of interest to be of sufficient relevance or weight to warrant exclusion from the committee. In brief, no committee members disclosed direct monetary or nonmonetary support from alcohol or pharmaceutical industry sources within the past 5 years, or that their clinical revenue would be influenced by the guideline recommendations.
To mitigate any real, potential or perceived risk of bias, 1 committee member who disclosed a direct potential competing interest involving employment with an addiction treatment organization was recused from voting on relevant recommendations. Additionally, 15 people disclosed special interests in relation to the guideline, pertaining to specific expertise, clinical experience, advisory board membership for nonprofit or community organizations, or research interests and publications, but the Competing Interest Adjudicator did not judge these to represent competing interests that precluded these individuals’ participation in committee activities.
Implementation
The release of this guideline follows the recent publication of Canada’s Guidance on Alcohol and Health,2 and it is hoped that both of these guidelines will collectively help to address the overall harms attributable to alcohol in Canada. In most Canadian jurisdictions, the lack of resources dedicated to care of substance use disorders, as well as the dearth of provincial or national comprehensive guidelines, have likely hindered the implementation of evidence-based treatments for AUD.
National and regional health policy-makers can substantially improve standards of care by promoting the adoption of this guideline and its recommendations. An example of a policy action that would facilitate providers in offering evidence-based medications would be to apply standard benefit status to all AUD medications on regional formularies, rather than requiring any form of special authorization.
With available resources, CRISM is pursuing a knowledge translation strategy to educate primary care providers, including efforts to track and measure implementation. Nevertheless, investments to reduce structural barriers (e.g., lack of physician training in addiction medicine) require policy-maker attention.7 Implementation efforts should also include low-barrier access points, as people with AUD, particularly those from marginalized communities, face multiple barriers to entering the traditional health care system.
Evidence for addressing high-risk drinking and AUD continues to evolve, and this guideline will be updated periodically as new knowledge becomes available.
Other guidelines
Several guidelines related to the management of high-risk alcohol use and AUD have been published in the last 5 years (Table 4). Generally, our recommendations are consistent with previous guidelines. A notable difference is that our guideline comprehensively addresses the full treatment pathway, including the issues of polypharmacy and the common use of medications that have poor evidence of benefit.
Other national and selected international guidelines considering alcohol use, high-risk drinking and alcohol use disorder published in the last 5 years
Gaps in knowledge
Importantly, support for evaluations of traditional wellness approaches used by Indigenous communities has been lacking, resulting in limited literature on these and other populations. Additionally, studies of psychosocial interventions have not used consistent approaches, durations or outcomes, making it difficult to determine the optimal strategies and lengths. Similarly, certain at-risk populations (e.g., incarcerated populations) and community-based approaches (e.g., residential treatment) have not received sufficient research attention and have not been studied in RCTs.
Most clinical trials have relatively small sample sizes and short-term durations (e.g., 8 weeks), with clinically relevant outcomes often not considered. For instance, in a systematic review of placebo-controlled randomized trials of SSRIs for depression reporting intermediate-term follow-up, the authors aimed to examine the impact of SSRI therapy on heavy drinking days and found that less than 10% of trials reported on alcohol use.61 Although there is a relatively high degree of consistent findings across naltrexone and acamprosate trials,18 studies of SSRIs are highly heterogenous, potentially as a result of underlying biases described elsewhere.62 Additionally, given that medications alone are unlikely to be effective in many instances of AUD, the role of commonly prescribed medications (e.g., naltrexone, SSRI) in potentially augmenting or undermining psychosocial treatment interventions similarly deserves further study.63,64
Finally, although meta-analyses suggest that certain psychotherapies or anticraving medications have good evidence for surrogate outcomes (e.g., preventing return to alcohol use),18 fewer studies include clinically relevant and longer-term health outcomes (e.g., morbidity, mortality).
Limitations
A limitation to the development process was the length of time between the initial literature search (September 2020) and the publication of this guideline. However, committee and reviewer comments often fuelled targeted literature searches that garnered more recent results, which were included in the text. In addition, a single individual assessed the quality of the included studies.
Importantly, the scope did not include comprehensive guidance for AUD with co-occurring substance use disorders or AUD comorbid with severe mental health conditions. Here, we suggest specialty consultation where available. Finally, although we developed a committee well positioned to identify and address the needs of primary care providers and the broad needs and perspectives of people affected by high-risk drinking and AUD, certain groups — including immigrant and refugee populations — were not represented.
Conclusion
Owing to the severe underutilization of evidence-based treatment approaches for high-risk drinking and AUD in Canada, in this guideline we promote expanded access to the full range of evidence-based treatments, including AUD pharmacotherapies (e.g., naltrexone, acamprosate) and psychosocial and mental health interventions, while also seeking to address the common occurrence of ineffective and potentially harmful prescribing and other practices. The aim of this guideline is to support primary care providers and services to offer more effective treatments routinely to patients with AUD as the standard of practice, with resulting improvements in health as well as potential for considerable cost savings in health and social systems.
Acknowledgements
The authors are thankful to all 36 committee members for their involvement as subject matter experts, the external peer reviewers, Canadian Research Initiative in Substance Misuse staff involved in the production of this guideline, Christa Ledding for her graphic design work and Maryam Babaei for assistance with article screening. Committee details are available in the full guideline (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230715/tab-related-content).
Footnotes
Competing interests: Evan Wood is a physician who works for Vancouver Coastal Health in the area of withdrawal management and undertakes work in the area of occupational addiction medicine. Dr. Wood is also a professor of medicine based at the University of British Columbia (UBC), a position supported by a Canadian Institutes of Health Research (CIHR) Tier 1 Canada Research Chair, and has received salary support from an R01 from the US National Institute on Drug Abuse, paid to UBC. Dr. Wood’s research lab is further supported by CIHR grants to the Canadian Research Initiative in Substance Misuse. Dr. Wood has also undertaken consulting work in legal matters related to substance use disorders and for a mental health company called Numinus Wellness, where Dr. Wood is former chief medical officer; Dr. Wood has also received compensation in the form of equity in Numinus. Dr. Wood reports receiving honoraria for non-industry related lectures and presentations (e.g., at academic or educational conferences), including a talk at the Canadian Society of Addiction Medicine (CSAM) paid by CSAM conference; a Rounds Presentation at Dalhousie University (paid by the University); and an educational talk for the allied health educational platform, Executive Links (all outside the submitted work and did not involve funding from the pharmaceutical industry). Dr. Wood has also received payment for expert reports and expert testimony in legal matters pertaining to substance use disorder, including from the Canadian Medical Protective Association and from trade unions representing workers with possible substance use disorder. Dr. Wood has received travel support from the CIHR. Jessica Bright, Nirupa Goel and Josey Ross report receiving salary support from the British Columbia Centre on Substance Use, in support of the present manuscript. Katelyn Halpape reports receiving grant funding from the Health Canada Substance Use and Addictions Program and Indigenous Services Canada for the University of Saskatchewan Chronic Pain Clinic. Dr. Halpape also reports receiving an honorarium as chapter editor of The Clinical Handbook of Psychotropic Drugs. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Evan Wood, Jessica Bright, Katrina Hsu, Nirupa Goel, Josey Ross, Averill Hanson and Jürgen Rehm contributed to the conception and design of the work; the acquisition, analysis and interpretation of data; and drafting the manuscript. Rand Teed, Ginette Poulin, Bryany Denning, Kim Corace, Corrina Chase, Katelyn Halpape, Ronald Lim and Tim Kealey contributed to the acquisition, analysis and interpretation of data. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This guideline was supported by the following: Health Canada (Substance Use and Addictions Program 2021-HQ-000066); Canadian Institutes of Health Research (CIHR), via Canadian Research Initiative in Substance Misuse (CRISM) Phase II: The Ontario Canadian Research Initiative Node Team (FRN 477887, Jürgen Rehm) and CRISM: BC Node (FRN 181674; Evan Wood); and CIHR via a Tier 1 Canada Research Chair in Addiction Medicine (Evan Wood).
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/













Podcast