Lymphocytic and collagenous colitis are forms of microscopic colitis that can be caused by drugs.
After re-exposure to a causative medication, such as entacapone, recurrence can be more acute and severe than after the initial exposure.
After identification of drug-induced lymphocytic colitis, stopping the offending medication is the first, and often most effective, step in management.
Lymphocytic colitis can be managed conservatively in mild cases, but therapy with budesonide may be necessary in more severe presentations.
An 84-year-old man with Parkinson disease presented to the emergency department with a 10-day history of watery, nonbloody diarrhea. He reported about 15–20 bowel movements per day, accompanied by urgency, episodes of incontinence and nocturnal diarrhea, but no pain. He reported substantial weakness and light-headedness when moving from lying to sitting or standing. He had no fever, vomiting or nausea, and no risk factors for infectious diarrhea (e.g., recent antibiotic use, sick contacts, travel history, relevant food exposures).
The patient’s symptoms began within 24 hours of his first dose of entacapone, an inhibitor of catechol-O-methyltransferase used in patients with Parkinson disease to inhibit the peripheral metabolism of levodopa. His neurologist had prescribed entacapone several years earlier, but the patient had developed diarrhea during therapy and the drug was stopped, with symptom resolution within days. In contrast, during this second trial of treatment, the patient took only 1 dose and diarrhea persisted for 10 days before his presentation to the emergency department.
On examination, the patient appeared hypovolemic, with flat jugular veins, a blood pressure of 110/70 mm Hg and a postural increase in heart rate of 32 beats/min. His abdomen was soft, with no notable tenderness or signs of peritonitis but hyperactive bowel sounds. Examination was otherwise unremarkable, except for features of Parkinsonism.
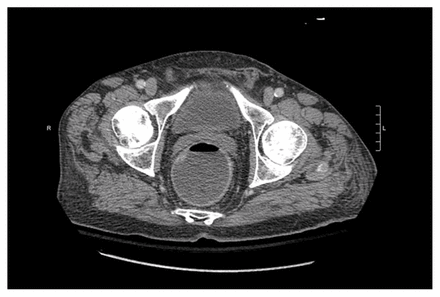
Blood tests showed a normal complete blood count and C-reactive protein level, severe hypokalemia at 1.8 (normal 3.5–5) mmol/L and mild renal insufficiency (creatinine 135 [normal 62–106] µmol/L, urea 7 [normal 2.5–10.7] mmol/L). At this point, the differential diagnosis for acute diarrhea included infectious or ischemic colitis, as well as an adverse drug reaction. An assay for Clostridium difficile toxin and cultures from stool samples were negative. To rule out a surgical cause for his presentation, the emergency department team ordered a computed tomography scan of the abdomen, which showed a fluid-filled colon with no bowel wall edema or stranding (Figure 1). The patient received fluid and electrolytes and was admitted to our general internal medicine service.
Computed tomography scan of the abdomen and pelvis of an 84-year-old man with lymphocytic colitis, showing a fluid-filled colon with no other signs of inflammation, such as mural thickening or fat stranding.
To provide symptomatic relief, we prescribed maximum doses of antidiarrheal and antisecretory medications (loperamide and octreotide) for 1 week while he waited for inpatient colonoscopy. He did not respond to these treatments.
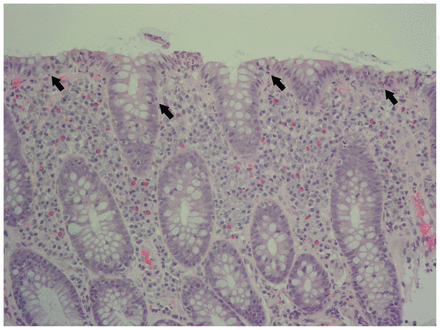
Colonoscopy showed a mildly edematous right colon, with no other abnormalities. The results of colonic biopsy specimens were available 2 days after colonoscopy; they showed prominent lymphoplasmacytic infiltrates with intraepithelial lymphocytosis and focal acute inflammation, but no notable architectural distortion, granulomas or thickening of the subepithelial collagen band (Figure 2).
Biopsy specimen from the ascending colon of an 84-year-old man with lymphocytic colitis (hematoxylin and eosin at ×20 magnification), showing intraepithelial lymphocytes (arrows) with relative sparing of crypt architecture and no thickening of subepithelial collagen band.
We diagnosed lymphocytic colitis and treated the patient with oral budesonide (9 mg/d). Within 24 hours, his bowel movements decreased to about 2 per day, and he was discharged shortly thereafter. Unfortunately, however, owing to his Parkinson disease, the patient fell a month after discharge and subsequently died.
Discussion
Acute-onset diarrhea (i.e., < 2 wk duration) is a common presenting complaint to the emergency department, with a wide differential diagnosis including infection, ischemia and adverse drug reactions.1 When stool studies are negative for infectious causes, endoscopic evaluation may be considered for patients in whom luminal inflammation is suspected; imaging can help rule out surgical causes.1 Antimotility medications, such as loperamide or diphrenoxylate–atropine, or other antidiarrheal medications (e.g., bismuth subsalicylate, octreotide) can be used immediately for symptomatic relief in those without signs of severe disease such as abdominal pain, fever, bloody stool.2 The presence of the latter may suggest severe colitis, for which antimotility medications may prolong the course of potential infection or lead to severe complications such as toxic megacolon.3
Lymphocytic and collagenous colitis are forms of microscopic colitis, a poorly understood inflammatory disease of the colon, which has an age- and sex-adjusted incidence of 21 per 100 000 person-years in North America.4 However, in patients who undergo colonoscopy for evaluation of chronic diarrhea (i.e., > 4 wk duration), microscopic colitis is diagnosed in 10%–20% of cases.5 Most cases of microscopic colitis occur in patients aged 50 years or older, and women are at higher risk than men for developing the collagenous subtype.6 No genetic or ethnic risk factors are clearly associated with the development of microscopic colitis.6
Patients with microscopic colitis most commonly present with a history of chronic watery diarrhea of varying severity. Severe cases are accompanied by hypovolemia, electrolyte abnormalities, abdominal pain, weight loss, fecal incontinence and challenges with activities of daily living.7 The mechanisms underlying microscopic colitis are largely unknown, although several hypotheses have suggested autoimmunity, bile acid malabsorption and dysfunction of the intestinal microbiome as contributing factors.7
Recent data suggest that the lymphocytic subtype of microscopic colitis can develop as an idiosyncratic hypersensitivity reaction to medications. The most commonly implicated drugs are nonsteroidal anti-inflammatory drugs, proton pump inhibitors and selective serotonin reuptake inhibitors.8 In our patient, the severity of diarrhea within hours of re-exposure to entacapone and the history of diarrhea with initial exposure suggests a prominent role of immunologic memory and is consistent with a diagnosis of drug-induced hypersensitivity reaction, with entacapone as the likely cause.
Although several medications have been associated with lymphocytic colitis, few meet causality criteria of the World Health Organization Uppsala Monitoring Centre (WHO-UMC) to be considered to be precipitating an adverse drug reaction.9 Entacapone is reported to cause diarrhea in 10%–20% of patients, and this is the most common reason for stopping the medication.10 Its association with lymphocytic colitis was first described in a series of 3 cases in France; in each case the condition resolved on stopping the medication.11 Our patient’s recurrence of severe diarrhea after drug re-challenge fulfils the WHO-UMC causality criteria, underscoring lymphocytic colitis as a certain adverse drug reaction to entacapone. The severity and persistence of the patient’s diarrhea despite stopping the drug also illustrates that drug-induced lymphocytic colitis sometimes requires corticosteroid therapy.
Colonoscopy typically shows a grossly normal appearance in patients with microscopic colitis. However, biopsies from the intestinal lumen show an increased number of intraepithelial lymphocytes with little or no disruption of crypt architecture.12 Thickening of the subepithelial collagen band is seen in collagenous colitis but not lymphocytic colitis.12 The presence of more than 20 intraepithelial lymphocytes per 100 surface epithelial cells, without thickening of the subepithelial collagen band, is diagnostic of lymphocytic colitis.12
Abdominal imaging is generally not required in the evaluation of microscopic colitis or other forms of chronic diarrhea, unless clinicians have specific concerns of surgical causes (e.g., obstruction, mass, fulminant colitis, abscess). Blood tests, including erythrocyte sedimentation rate and C-reactive protein, and fecal calprotectin are not reliable markers of microscopic colitis.12
Management of drug-induced microscopic colitis requires stopping the precipitating medication, which is usually effective.13 Loperamide and other antidiarrheal medications, such as bismuth subsalicylate, may be effective for persistent diarrhea in patients with mild microscopic colitis. Cholestyramine may also be effective, supporting the hypothesis that bile acid malabsorption may be implicated in the pathogenesis.12 In more severe disease, evidence supports budesonide as a highly effective treatment, with remission rates of greater than 80%.9 A treatment guideline has recently been published by the American Gastroenterological Association.14
A typical course of budesonide for treatment of microscopic colitis is 9 mg/d for 8 weeks,14 although some patients require a gradual taper over months to avoid recurrent symptoms.12 If biopsy-proven microscopic colitis does not respond to budesonide, other causes of diarrhea (e.g., celiac disease) should be ruled out. In patients with recurrent disease, budesonide is indicated for both reinduction and maintenance of remission.14 We had to establish the diagnosis of microscopic colitis before starting therapy in our patient; however, a patient with typical recurrence of symptoms after an established diagnosis can be treated empirically if other appropriate causes have been excluded (e.g., infectious colitis).
No randomized controlled trials have been published supporting the use of immunomodulatory therapy and antitumour necrosis factor (anti-TNF) therapy in drug-induced lymphocytic colitis; however, case reports suggest azathioprine or anti-TNF therapy may be effective and may warrant consideration by a specialist in corticosteroid-resistant cases.7
Although lymphocytic colitis is generally considered a chronic diarrheal illness, severe drug-induced lymphocytic colitis can present acutely, especially after previous sensitization. Drug-induced lymphocytic colitis should be considered in any patient who has recently started a new medication that has been associated with the diagnosis. Given increasing use of entacapone, lymphocytic colitis should be considered in patients who develop diarrhea during the initial period of treatment with this agent.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: David Rodrigues and David Juurlink conceived and designed the work. Michael Bernstein performed the diagnostic procedure and Eugene Hsieh provided the pathological analysis. David Rodrigues and David Juurlink drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/