Abstract
Background: Higher doses of opioids, mental health comorbidities, co-prescription of sedatives, lower socioeconomic status and a history of opioid overdose have been reported as risk factors for opioid overdose; however, the magnitude of these associations and their credibility are unclear. We sought to identify predictors of fatal and nonfatal overdose from prescription opioids.
Methods: We systematically searched MEDLINE, Embase, CINAHL, PsycINFO and Web of Science up to Oct. 30, 2022, for observational studies that explored predictors of opioid overdose after their prescription for chronic pain. We performed random-effects meta-analyses for all predictors reported by 2 or more studies using odds ratios (ORs) and 95% confidence intervals (CIs).
Results: Twenty-eight studies (23 963 716 patients) reported the association of 103 predictors with fatal or nonfatal opioid overdose. Moderate- to high-certainty evidence supported large relative associations with history of overdose (OR 5.85, 95% CI 3.78–9.04), higher opioid dose (OR 2.57, 95% CI 2.08–3.18 per 90-mg increment), 3 or more prescribers (OR 4.68, 95% CI 3.57–6.12), 4 or more dispensing pharmacies (OR 4.92, 95% CI 4.35–5.57), prescription of fentanyl (OR 2.80, 95% CI 2.30–3.41), current substance use disorder (OR 2.62, 95% CI 2.09–3.27), any mental health diagnosis (OR 2.12, 95% CI 1.73–2.61), depression (OR 2.22, 95% CI 1.57–3.14), bipolar disorder (OR 2.07, 95% CI 1.77–2.41) or pancreatitis (OR 2.00, 95% CI 1.52–2.64), with absolute risks among patients with the predictor ranging from 2–6 per 1000 for fatal overdose and 4–12 per 1000 for nonfatal overdose.
Interpretation: We identified 10 predictors that were strongly associated with opioid overdose. Awareness of these predictors may facilitate shared decision-making regarding prescribing opioids for chronic pain and inform harm-reduction strategies
Systematic review registration: Open Science Framework (https://osf.io/vznxj/)
Chronic pain affects 20% of the population worldwide1–5 and is commonly managed with opioids. A 2021 systematic review of 60 observational studies found that opioids are prescribed for 27% of adults living with chronic pain, with higher prevalence of prescribing in North America than in Europe.6 In 2018, 13% of people in Canada (aged ≥ 15 yr) reported use of an opioid analgesic in the past year.7 Opioid use is associated with serious harms, including addiction, and nonfatal and fatal overdose.8
From January to September 2022, 5360 deaths were attributed to opioid toxicity in Canada.9 Although determining the relative contribution of prescribed and illicit opioids is complex, a study of 2910 opioid-related deaths in Ontario, Canada, found that, in 2016, one-third of those who died had an active opioid prescription and more than 75% had been dispensed an opioid within 3 years of death.10 Prescription opioid use has been associated with illicit drug use. A cohort study of 59 804 adults in British Columbia, Canada, found that patients who were prescribed opioids for noncancer pain were 8 times more likely to start injection drug use than opioid-naïve patients.11
Several systematic reviews have explored predictors for fatal and nonfatal opioid overdose following prescription for chronic pain.12–17 The most consistently reported associations with opioid overdose were higher doses of opioids, mental health comorbidities, co-prescription of sedatives, lower socioeconomic status and a history of opioid overdose (Appendix 1, eTable 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230459/tab-related-content). However, these reviews have important limitations, including lack of statistical pooling of measures of association,12,13,15–17 inadequate assessment of risk of bias,12,15,16 outdated searches,12–17 a focus on select populations such as older adults12 or people who have been incarcerated,13 and failure to evaluate the overall certainty of evidence.12–17 We sought to identify predictors of fatal and nonfatal overdose after prescription of opioids for chronic pain that addresses these limitations.
Methods
In conducting our systematic review and meta-analysis we followed the reporting of Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement,18 as well as guidance for systematic review and meta-analysis of prognostic studies,19 and registered our protocol at Open Science Framework (https://osf.io/vznxj/).
Data sources and searches
A medical research librarian performed database-specific electronic searches of MEDLINE, Embase, CINAHL, PsycINFO and Web of Science from inception to Oct. 30, 2022, without language restrictions (Appendix 1, Section 1). We screened reference lists of all eligible studies and 6 previous reviews12–17 for additional studies.
Study selection
We included cohort or case–control studies that explored, in an adjusted analysis, predictors for fatal or nonfatal opioid overdose after prescription of opioids for chronic noncancer or cancer-related pain lasting 3 or more months. Eligible studies had to provide explicit statements that they followed a patient population in which at least 80% were prescribed opioids for chronic pain, for which the date of the first prescription for opioids was known, and fatal or nonfatal overdose was an outcome that was assessed.
We excluded studies that enrolled palliative care patients or exclusively patients who had previously had an opioid overdose. Studies were also ineligible if they included, in all available models, significant associations with variables collected after baseline because these variables may be a result, rather than a cause, of opioid overdose. When study populations overlapped by more than 50% between articles, we included only data in the largest study. We contacted authors to clarify eligibility or to acquire missing data. Four pairs of reviewers independently screened the titles and abstracts of identified citations and the full texts of potentially eligible studies.
Data extraction and risk of bias assessment
Using standardized, pilot-tested data extraction forms and a detailed instruction manual (available at https://osf.io/vznxj/), 4 pairs of reviewers extracted data and assessed the risk of bias from all eligible studies, independently and in duplicate. We collected information regarding study and patient characteristics, and measures of association for all reported predictors. Reviewers addressed discrepancies through discussion, or adjudication by a third reviewer (L.W.), when necessary.
We assessed risk of bias by evaluating the representativeness of the study population, validity of outcome assessment, loss to follow-up and whether predictive models were adjusted, at minimum, for age, sex, substance use disorder and any other comorbid mental illness. We modified the assessment criteria from the Users’ Guides to the Medical Literature (Appendix 1, Section 2).20
Data analysis
We used the κ statistic to measure inter-rater agreement for full-text screening.21
We pooled the prevalence of nonfatal or fatal overdose using random-effects models with a Freeman–Tukey Double Arcsine transformation to stabilize the variance.22
When possible, we pooled all predictors associated with opioid overdose that were reported by at least 2 studies as odds ratios (ORs) and 95% confidence intervals (CIs). When studies provided adjusted relative risks (RRs) or hazard ratios (HRs), we pooled them with ORs, given the low baseline risk of fatal (1 in 100023) and nonfatal (2 in 100024) overdose.25 We performed random-effects models using the DerSimonian–Laird method for all meta-analyses of 3 or more studies, and fixed-effects models when pooling 2 studies.26 We pooled predictors for opioid overdose in general, as we did not find credible subgroup effects between fatal and nonfatal opioid overdose.
When eligible studies explored associations between individual types of opioids versus all other types of opioids and overdose,27,28 we considered only the comparison of fentanyl versus non-fentanyl opioids, given concerns regarding an elevated risk of overdose with fentanyl because of its high potency.10
To evaluate the relationship of morphine equivalent dose with opioid overdose, we performed a 2-stage, random-effects, dose–response meta-analysis.29,30 We also performed a 1-stage dose–response meta-analysis as a sensitivity analysis.31 When associations for age were reported as categorical data, we converted into continuous data.32–34
We considered relative associations to be large when the pooled OR was 2.0 or higher, or 0.5 or less, and small-to-trivial when the pooled OR was greater than 0.5 and less than 2.0.
We explored the consistency of associations between our pooled results and studies reporting the same predictors that were not possible to pool (e.g., the authors reported the association was significant, but with no accompanying data). We deemed predictors as promising if they were not amenable to meta-analysis but single studies reported a highly significant (p ≤ 0.001) and large association (OR ≥ 2.0 or OR ≤ 0.5) with a study population of at least 1000 patients.34
To avoid overestimating associations, we imputed an OR of 1 for predictors that were excluded in adjusted analyses because of nonsignificance.34,35 We calculated the absolute risk for each predictor amenable to meta-analysis to facilitate interpretation using baseline risks of 1 in 100023 for fatal overdose and 2 in 100024 for nonfatal overdose. We used Stata statistical software version 17.0 (StataCorp) for all analyses.
Small study effects
When at least 10 studies were included in a meta-analysis,33,36 we assessed publication bias by funnel plots and the Egger test.37
Subgroup analyses, meta-regression and sensitivity analyses
We evaluated heterogeneity using forest plots36 and τ2 for all random-effects models. We conducted a priori subgroup analyses for factors that we hypothesized would have a larger association with opioid overdose among chronic noncancer versus cancer-related pain; fatal versus nonfatal overdose; intentional versus unintentional overdose; high versus low risk of bias on a component-by-component basis; current versus previous substance use disorder; comorbid mental health disorders versus none; co-prescription of benzodiazepines versus no benzodiazepine prescription; and tobacco use disorder versus current tobacco use. We conducted subgroup analyses if each subgroup contained at least 2 studies. We assessed the credibility of significant effects using Instrument for Assessing the Credibility of Effect Modification Analyses (ICEMAN) criteria.38
We performed sensitivity analyses by excluding studies for which we imputed an OR of 1, converted categorical data to continuous data or derived measures of association from the RR or HR, and studies that did not exclusively enroll patients with chronic pain.
Certainty of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarize the certainty of evidence.36 We rated down for imprecision if the 95% CI included both a small-to-trivial and large association with opioid overdose. Given the number of predictors considered in our review, we focused our main presentation of findings on predictors that showed large relative associations (OR ≥ 2 or ≤ 0.5) with opioid overdose supported by moderate- or high-certainty evidence.
Ethics approval
We did not seek ethics approval for this systematic review and meta-analysis of published data.
Results
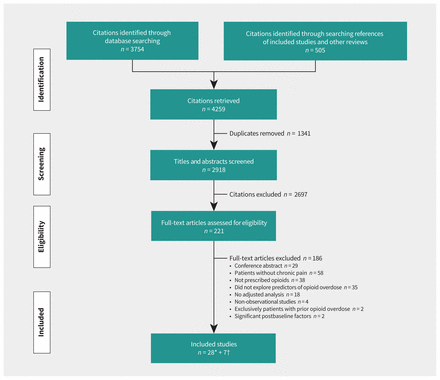
We reviewed 2918 citations and included 28 unique studies in our analyses (21 cohort studies23,24,39–57 and 7 case-control studies27,28,58–62). We also identified 7 studies (4 cohort63–66 and 3 case-control studies67–69) with overlapping populations that reported unique predictors (Figure 1 and Appendix 1, Section 3 and eTable 1). Inter-rater agreement for full-text screening was near perfect (κ = 0.89). We contacted 64 authors; 2542,44,46,50,52,56,61,69–86 of 33 authors clarified eligibility, and 1024,46,47,51,55,63,65–67,78 of 31 authors provided additional data for meta-analysis.
Flow diagram of study selection. *Twenty-eight studies with the largest sample size and longest follow-up were included in our primary analysis; among these, 3 articles reported 2 separate cohorts.46,53,58 †Seven studies63–69 included overlapping study populations with 4 studies55,56,58,60 included in our primary analysis.
Study characteristics
The 28 studies enrolled a total of 23 963 716 patients (52% female) with a median of the mean age of 52 years (interquartile range [IQR] 47 to 57). Twenty-four studies were conducted in the United States, 3 were conducted in Canada and 1 was conducted in the United Kingdom. Twenty-one studies23,24,40–47,50–53,55–57,59–62 included only patients with chronic noncancer pain, and 727,28,39,48,49,54,58 included patients with either chronic noncancer or cancer-related pain. Twenty-two studies enrolled patients with previous or current substance use disorder (median proportion 9%, IQR 4%–13%),23,24,27,28,39–43,46,48–51,53–55,57,58,60–62 and 3 studies excluded patients with comorbid substance use disorder.45,52,56 Twenty-three studies included patients with comorbid mental illness (median proportion 31%, IQR 20%–41%),23,24,27,28,40–42,45–49,51–58,60–62 and 5 studies exclusively recruited veterans.27,40,51,55,58 All studies used administrative databases. The median sample size was 43 885 (IQR 11 186–203 353) (Table 1 and Appendix 1, eTable 2).
Characteristics of 28 eligible studies
Risk of bias
Twenty-five studies (89%) were at high risk of bias for at least 1 criterion (Appendix 1, eTable 3). Thirteen studies (46%) included samples that were not representative of the target study population, because all patients were veterans,27,40,51,55,58 more than 60% were patients with comorbid mental illness,41,48,61 all patients were aged 65 years or older,62 more than 50% of patients were disabled44 or patients were prescribed a high daily opioid dose (≥ 50 mg54,56 or ≥ 90 mg morphine equivalent).49 Seventeen studies (61%) were unable to exclude illicit opioid overdoses,24,27,39–42,46,48–50,52–57,61 which may compromise the validity of the outcome measure. Seventeen studies (61%) reported adequately adjusted regression models.23,24,27,28,40,41,44–46,49,52,53,55,58,60,61 Only 3 studies reported loss to follow-up (all < 20%)27,40,50 (Appendix 1, eTable 3).
Predictors of fatal and nonfatal overdose
Moderate-certainty evidence showed the pooled prevalence of fatal opioid overdose after prescription for chronic pain was 1.3 per 1000 (95% CI 0.6–2.3 per 1000) for fatal overdose and 3.2 per 1000 (95% CI 2.0–4.7 per 1000) for nonfatal overdose (Appendix 1, eFigure 1A, eFigure 1B and eTable 4). A total of 103 predictors associated with opioid overdose were reported, among which 72 were amenable to meta-analysis.
Opioid prescribing predictors
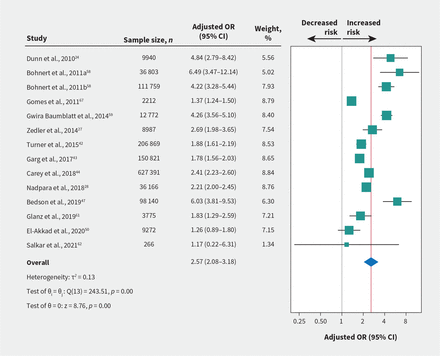
High-certainty evidence from 14 studies involving 1 315 173 patients showed a linear dose–response relationship with opioid overdose (Appendix 1, eFigure 2A and eTable 5). The association was small at a 50-mg morphine equivalent dose/day (OR 1.69, 95% CI 1.50–1.90; Appendix 1, eFigure 2B and eTable 6) and large at 90 mg (OR 2.57, 95% CI 2.08–3.18; Figure 2 and Table 2), with an absolute risk 2.6 per 1000 for fatal overdose and 5.1 per 1000 for nonfatal overdose at a 90-mg morphine equivalent dose/day.
Association of prescribed opioid dose (per 90-mg morphine equivalent dose/d increase) with risk of fatal or nonfatal opioid overdose. Bohnert et al., 2011a is for 36 803 patients with chronic cancer pain; Bohnert et al., 2011b is for 111 759 patients with chronic noncancer pain. Note: CI = confidence interval, OR = odds ratio.
Evidence profile of predictors with large associations with fatal or nonfatal overdose following opioid prescription for chronic pain
Moderate- to high-certainty evidence showed large associations between opioid overdose and 3 or more prescribers (OR 4.68, 95% CI 3.57–6.12), 4 or more dispensing pharmacies (OR 4.92, 95% CI 4.35–5.57) and prescription of fentanyl versus other opioids (OR 2.80, 95% CI 2.30–3.41). The absolute risks ranged from 2.8 to 4.9 per 1000 for fatal overdose, and from 5.6 to 9.8 per 1000 for nonfatal overdose (Appendix 1, eFigures 2C–2E and Table 2).
Moderate- to high-certainty evidence showed small-to-trivial increased risks of opioid overdose with long- versus short-acting opioid formulations; number of naloxone prescriptions; as-needed and regularly scheduled versus scheduled administration alone; and longer versus shorter duration of opioid use. The absolute risks ranged from 1.0 to 1.9 per 1000 for fatal overdose, and from 2.0 to 3.8 per 1000 for nonfatal overdose (Appendix 1, eFigure 2F, eFigure 2G and eTable 6).
Co-prescription predictors
Moderate-certainty evidence suggested small associations between opioid overdose and co-prescription of benzodiazepines, anticonvulsants, sedatives or muscle relaxants, with the absolute risks ranging from 1.3 to 1.8 per 1000 for fatal overdose, and from 2.6 to 3.6 per 1000 for nonfatal overdose (Appendix 1, eFigures 3A–3D and eTable 7).
Psychological predictors
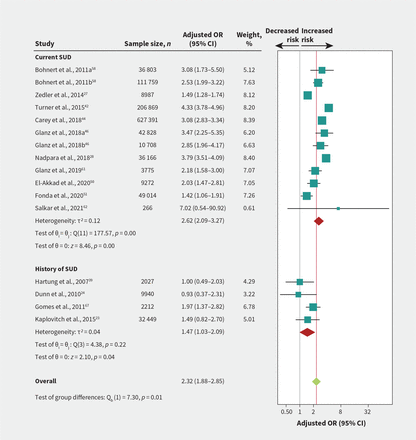
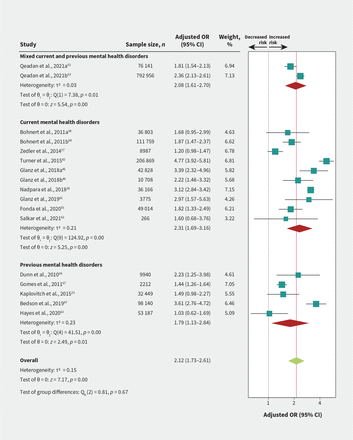
We found a credible subgroup effect between current versus previous substance use disorder (p = 0.01; Figure 3 and Appendix 1, Section 4); therefore, we reported results for these predictors separately. Moderate- to high-certainty evidence showed large associations between opioid overdose and current substance use disorder (OR 2.62, 95% CI 2.09–3.27; Figure 3), any mental health diagnosis (OR 2.12, 95% CI 1.73–2.61; Figure 4), depression (OR 2.22, 95% CI 1.57–3.14; Appendix 1, eFigure 4A), and bipolar disorder (OR 2.07, 95% CI 1.77–2.41; Appendix 1, eFigure 4B). The absolute risks ranged from 2.1 to 2.6 per 1000 for fatal overdose, and from 4.1 to 5.2 per 1000 for nonfatal overdose (Table 2).
Association of substance use disorder (SUD) and risk of fatal or nonfatal opioid overdose. Test of interaction = 0.01 for current versus history of SUD. Bohnert et al., 2011a is for 36 803 patients with chronic cancer pain; Bohnert et al., 2011b is for 111 759 patients with chronic noncancer pain. Glanz et al., 2018a is for 42 828 patients from the derivation site cohort; Glanz et al., 2018b is for 10 708 patients from the validation site cohort. Note: CI = confidence interval, OR = odds ratio.
Association of comorbid mental illness and risk of fatal or nonfatal opioid overdose Test of interaction = 0.67. Bohnert et al., 2011a is for 36 803 patients with chronic cancer pain; Bohnert et al., 2011b is for 111 759 patients with chronic noncancer pain. Glanz et al., 2018a is for 42 828 patients from the derivation site cohort; Glanz et al., 2018b is for 10 708 patients from the validation site cohort. Qeadan et al., 2021a is for 76 141 patients with chronic pain syndrome; Qeadan et al., 2021b is for 792 956 patients with low back pain. Note: CI = confidence interval, OR = odds ratio.
Moderate-certainty evidence suggested smaller associations with psychotic disorders, tobacco use or tobacco use disorder, history of substance use disorder and anxiety. The absolute risks ranged from 1.3 to 1.6 per 1000 for fatal overdose, and from 2.6 to 3.2 per 1000 for nonfatal overdose (Appendix 1, eFigures 4C–4E and eTable 8).
Medical predictors
Moderate- to high-certainty evidence showed large associations between opioid overdose and history of overdose (OR 5.85, 95% CI 3.78–9.04; absolute risk 5.9 per 1000 for fatal overdose, 11.7 per 1000 for nonfatal overdose; Appendix 1, eFigure 5A) and pancreatitis (OR 2.00, 95% CI 1.52 to 2.64; absolute risk 2.0 per 1000 for fatal overdose, 4.0 per 1000 for nonfatal overdose; Appendix 1, eFigure 5B) (Table 2).
Moderate- to high-certainty evidence showed small-to-trivial associations with heart failure, hemiplegia or paraplegia, renal disease, liver disease, chronic obstructive pulmonary disease, cancer, hypertension, diabetes, injury or acute pain, emergency department visit and higher Charlson Comorbidity Index scores. The absolute risks ranged from 1.1 to 1.7 per 1000 for fatal overdose, and from 2.3 to 3.3 per 1000 for nonfatal overdose (Appendix 1, eTable 9).
Sociodemographic predictors
Moderate- to high-certainty evidence showed small-to-trivial associations between opioid overdose and public or no insurance versus private insurance, White race or ethnicity versus other racial or ethnic groups, younger age, male sex, unmarried status and geographical region. The absolute risks ranged from 1.1 to 1.8 per 1000 for fatal overdose, and from 2.1 to 3.6 per 1000 for nonfatal overdose (Appendix 1, eFigures 6A–6F and eTable 10).
Predictors not amenable to pooling
The results from studies that reported predictors that we subjected to meta-analysis but whose data could not be included were consistent with our pooled analyses (Appendix 1, eTable 11). We were unable to pool 31 predictors that were each reported by a single study, of which 3 met our criteria as promising for future study, namely opioid tapering, opioid discontinuation (Appendix 1, eTable 12) and traumatic brain injury (Appendix 1, eTable 13). Twenty-three of 31 predictors were consistently not associated with opioid overdose (Appendix 1, eTable 14).
Additional analyses
No additional subgroup analysis or meta-regression were judged as credible according to ICEMAN criteria (Appendix 1, eTable 15). Our sensitivity analyses found no important differences in results whether we incorporated missing data for nonsignificant predictors, pooled different measures of associations (OR, RR or HR), included studies that did not exclusively enroll patients with chronic pain or converted categorical data on age to continuous data (Appendix 1, eTable 16). We detected no evidence of publication bias among predictors reported by at least 10 studies (Table 2 and Appendix 1, eFigure 7 and eTables 6–10).
Interpretation
In this systematic review of observational studies involving nearly 24 million patients receiving opioids for chronic pain, we pooled data on 72 predictors; of these, moderate- to high-certainty evidence showed large relative associations of opioid overdose with previous opioid overdose, current substance use disorder, depression, bipolar disorder, any mental health diagnosis, pancreatitis, 3 or more opioid prescribers, 4 or more dispensing pharmacies, prescribing 90-mg morphine equivalents or more, or prescription of fentanyl. We explored 31 additional predictors that could not be statistically pooled, and preliminary evidence suggested that opioid tapering or discontinuation strategies and traumatic brain injury warrant additional study.
Of the 6 previous systematic reviews that have explored predictors for opioid overdose,12–17 only 1 conducted meta-analysis for the single predictor of opioid dose.14 This review pooled 7 cohort studies and reported a larger association than our review (RR 4.28 for > 100 v. ≤ 100 morphine equivalent dose/d); however, they included unadjusted data that may overestimate associations.14 Clinical guidelines recommend against use of high doses (e.g., ≥ 90-mg morphine equivalent dose/d) when starting a trial of opioids for chronic pain management and recommend approaching patients on high doses to consider tapering to reduce potential harms, including overdose.8,87,88 However, forced or aggressive tapering of opioids or stopping opioids may increase risk of overdose and death.89,90 Our review found conflicting evidence from 6 studies,48,55,56,64–66 with 2 reporting that tapering or stopping decreased risk of overdose55,65 and 4 reporting no association or increased risk (Appendix 1, eTable 12).48,56,64,66 One source of this variability may be how tapering is approached. Emerging evidence suggests that voluntary, supported opioid tapering may help most people who are prescribed high-dose opioid therapy for chronic pain to safely reduce their dose.91,92 A clinical trial of 608 patients prescribed strong opioids to manage chronic noncancer pain, randomized to usual care or education and support for tapering, found that 29% of patients in the intervention arm had stopped opioids at 1 year, compared with 7% of patients in the usual care arm, but with no effect on perceived pain interference with daily life activities.93
Our review, which included 22 studies that were not considered by previous reviews,28,39,41,43–45,49–51,53–57,59,60,63–67,69 quantified large associations between opioid overdose and higher opioid dose,12,14–16 prescription of fentanyl,15 current substance use disorder, 12,15,16 mental health disorders,12 depression16,17 and previous opioid overdose.15 Previous systematic reviews have qualitatively summarized these associations, but with conflicting results.12,13,15–17 Further, we also found moderate- to high-certainty evidence for 33 additional predictors that were not reported by previous reviews.
We found large associations between opioid overdose and multiple prescribers or dispensing pharmacies, both of which have been linked to diversion and opioid use disorder.94,95 A cross-sectional study of nearly 1.5 million opioid prescriptions found that prescriber and pharmacy shopping accounted for 0.6% of dispensed medications.96 Prescription drug monitoring programs have been developed to address this issue, but their effectiveness in reducing opioid-related harms is uncertain.97,98 We found a large association between opioid overdose and pancreatitis, which may be a surrogate for alcohol use disorder.99 We found a smaller positive association between opioid overdose and number of naloxone prescriptions, which is likely a surrogate for patients at higher risk for opioid overdose.100
The opioid crisis has generated interest in identifying patients at higher risk of addiction or overdose and has led to the development of several screening tools; however, these instruments have either not been validated or have shown poor psychometric properties.46,101–103 Our findings suggest that awareness of, and attention to, several patient and prescription characteristics, may help reduce the risk of opioid overdose among people living with chronic pain.
Evidence alone is insufficient for clinical decisions regarding management of chronic pain, which also requires consideration of individual patient values. A systematic review found that people living with chronic pain place less value on the possibility of addiction versus the possibility of important pain relief.104 Our findings should prove helpful for conveying risks of overdose to patients when deciding whether to start a trial of opioids for chronic pain, and will facilitate evidence-based, shared decision-making.
Limitations
We were unable to pool data for predictors from studies that used different measures. Results from these studies were, however, consistent with results from studies amenable to pooling. Although we did not find credible subgroup effects for fatal versus nonfatal overdose, intentional versus unintentional overdose or chronic cancer versus noncancer pain, our analyses may have been underpowered as most eligible studies reported mixed types of opioid overdose and enrolled patients with both cancer and noncancer chronic pain.
Although opioid overdose is a serious outcome, we defined an important increase in risk as at least twice the baseline risk (i.e., OR ≥ 2) given that fatal and nonfatal overdoses are uncommon events. We reported all absolute measures of association and 95% CIs for predictors to facilitate use of alternate thresholds. Studies eligible for our review used administrative data to identify opioid-related overdose or death; however, this approach has shown more than 80%–90% positive predictive value.105,106 We did not assess the validity of predictor assessment; however, information on predictors was abstracted from either medical records, pharmacy, insurance or other administrative databases. As such, they are at low risk of bias for ascertainment of exposure.107
No opioid-conversion method for calculating the morphine equivalent dose is universally accepted, and competing approaches can yield important differences;108 however, 14 studies of more than 1 million patients provided high-certainty evidence for an association between higher doses of prescribed opioids and increased risk of overdose. Finally, we evaluated all included study models for whether they adjusted for age, sex, substance use disorder and any other comorbid mental illness; however, residual or unmeasured confounding may have affected our findings.
Conclusion
In this meta-analysis of observational studies of patients prescribed opioids for chronic pain, moderate- to high-certainty evidence showed large associations of fatal and nonfatal overdose with a history of opioid overdose, depression, bipolar disorder, a mental health diagnosis, current substance use disorder, pancreatitis, multiple opioid prescribers or dispensing pharmacies, prescription of 90-mg morphine equivalents or higher and prescription of fentanyl. Awareness of these predictors may facilitate shared decision-making regarding prescribing opioids for chronic pain and may inform harm-reduction strategies.
Acknowledgements
The authors thank Dr. Norman Buckley, Department of Anesthesia, McMaster University, for providing suggestions on clarification of opioid types and use of methadone for pain or for substance use disorder, and Dr. Alka Kaushal, Department of Family Medicine, McMaster University for literature screening. They thank Dr. Matthew Miller, Department of Health Sciences and Epidemiology, Northeastern University; Drs. Almut G. Winterstein and Yan Li, Department of Pharmaceutical Outcomes and Policy, College of Pharmacy, University of Florida; Dr. Joseph A Boscarino, Center for Health Research, Geisinger Clinic; Dr. Barbara J. Turner, Gehr Center for Health Systems Science and Innovation, Keck School of Medicine; Dr. Yuanyuan Liang, Department of Epidemiology and Public Health, University of Maryland School of Medicine; Dr. Alicia Agnoli, Department of Family and Community Medicine, University of California; Dr. Fusheng Wang, Department of Biomedical Informatics, Stony Brook University; Dr. Elizabeth Oliva, Veterans Affairs Program Evaluation and Resource Center, Veterans Affairs Office of Mental Health and Suicide Prevention; Dr. Krista Highland, Defense and Veterans Center for Integrative Pain Management, Department of Anesthesiology, Uniformed Services University; and Dr. Bradley C. Martin, Division of Pharmaceutical Evaluation and Policy, College of Pharmacy, University of Arkansas for Medical Sciences, for clarifying issues regarding their studies. They thank Dr. Tara Gomes, Li Ka Shing Knowledge Institute; Dr. Barbara J. Turner, Gehr Center for Health Systems Science and Innovation, Keck School of Medicine, University of Southern California; Dr. Michael Von Korff, Kaiser Permanente Washington Health Research Institute; Dr. John Bedson, Arthritis Research UK Primary Care Centre, Research Institute for Primary Care & Health Sciences, Keele University; Dr. Ying Chen, Biostatistics & Epidemiology, Department of Health and Environmental Sciences, Xi’an Jiaotong-Liverpool University; and Dr. Bradley C. Martin, Division of Pharmaceutical Evaluation and Policy, College of Pharmacy, University of Arkansas for Medical Sciences, for providing additional data. No financial compensation was provided to any of these individuals. We regret to inform readers that Dr. Boscarino has recently deceased.
Footnotes
Competing interests: Corey Hayes reports support from the National Institute on Drug Abuse Clinical Trials Network (5UG1DA013732-23S3). David Juurlink is an unpaid member of Physicians for Responsible Opioid Prescribing. David Juurlink is also a member of the American College of Medical Toxicology. David Juurlink has received payment for lectures from Texas Tech University Health Sciences Centre and payment for expert testimony regarding analgesics, including opioids. He has received travel support for presentations and scientific meetings from the Canadian Institutes of Health Research, Stanford University and Texas Tech University Health Sciences Centre. Jason Busse is the Principal Investigator for the update of the 2017 Canadian Opioid Guideline, which is funded by Health Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Li Wang and Jason Busse conceived and designed the study. Li Wang, Patrick Hong, Wenjun Jiang, Yasir Rehman, Brian Hong, Rachel Couban and Chunming Wang acquired the data. Li Wang carried out the statistical analysis. Li Wang, Corey Hayes, David Juurlink and Jason Busse interpreted the data. Li Wang and Jason Busse drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by a grant from Health Canada’s Substance Use and Addictions Program. Corey Hayes was supported by Veterans Affairs Health Services Research and Development under a Career Development Award-2 grant. Jason Busse is supported, in part, by a Canadian Institutes of Health Research Canada Research Chair in the prevention and management of chronic pain.
Data sharing: Details of the characteristics of the included studies were shared in the supplementary materials. The study protocol is available from the Open Science Framework (https://osf.io/vznxj/). Statistical codes and data can be obtained from the corresponding author.
- Accepted September 18, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/