Renal denervation (RDN) is a minimally invasive catheter-based intervention that uses 1 of a range of techniques to disrupt renal afferent and efferent nerve signals between the kidney and brain.
Renal denervation reduces office systolic blood pressure by an average of 4–6 mm Hg more than control patients and is proposed as an adjunctive treatment to pharmacotherapy for patients with uncontrolled hypertension.
Serious complications from renal denervation are uncommon, and minor access site complications occur in around 5% of patients.
Renal denervation is best provided by a multidisciplinary team including hypertension specialists and interventionalists.
In 2021, about 5.8 million people in Canada were living with hypertension.1 Despite the availability of pharmacotherapy, hypertension is a leading cause of death and disability globally, and in Canada, rates of treatment and control are declining.1 Controlled hypertension is defined as an office blood pressure (BP) of less than 140/90 mm Hg.1 Currently, 1 in 3 people in Canada living with hypertension has uncontrolled BP.1 Causes of lack of control include nonadherence to medications, adverse effects of medications, complex treatment regimens, physician inertia and resistant hypertension.2
A meta-analysis of 48 studies involving nearly 350 000 patients showed that a seemingly modest drop in systolic BP of 5 mm Hg over 4 years of follow-up lowered the risk of major cardiovascular events by 10%;3 the greater the antihypertensive effect, the greater the benefit on cardiovascular outcomes.
What is renal denervation?
The kidneys have a dense nerve supply, and previous studies have established the role of the sympathetic nervous system in regulating kidney function and BP.4,5 Among patients with uncontrolled hypertension, sympathetic nervous system overdrive causes increased renal renin excretion, which results in increased blood volume and arterial tone.4 The afferent and efferent renal nerves travel in close proximity to the renal arteries in the perivascular adipose tissue.
Renal denervation (RDN) is a minimally invasive catheter-based intervention that uses 1 of a range of techniques to disrupt renal afferent and efferent nerve signals between the kidney and brain. Renal denervation reduces office systolic BP by an average of 4–6 mm Hg more than control patients and is proposed as adjunctive treatment to pharmacotherapy for patients with uncontrolled hypertension.5
How is treatment delivered?
The procedure is performed by an experienced interventionalist under fluoroscopic guidance in an interventional catheterization suite. With the patient under deep conscious sedation, the RDN catheter is advanced to the renal arteries via the common femoral artery.
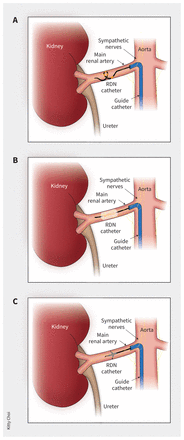
Several RDN technologies exist. The multielectrode RDN catheter (e.g., Medtronic) simultaneously delivers 60 seconds of radiofrequency energy to 4 gold electrodes in contact with the arterial wall in spiral configuration (Figure 1A).4 To minimize thermal effects, the design permits continuous blood flow during energy delivery, ensuring both arterial and electrode cooling during treatment. All accessible renal arteries with a diameter of 3–8 mm, including branch vessels and accessory arteries, are treated.4
Circumferential ablation of renal sympathetic nerves by (A) multielectrode radiofrequency ablation, (B) ultrasound-based denervation or (C) alcohol-mediated perivascular renal denervation. Radiofrequency treatment is delivered bilaterally in a number of locations along the main and extra-parenchymal renal arteries ranging from 3 to 8 mm in diameter. For ultrasound-based or alcohol-mediated denervation, treatment is applied to both main renal arteries only. Note: RDN = renal denervation.
Kitty Choi
The ultrasound RDN system (e.g., ReCor Medical) delivers ultrasound energy to thermally ablate the renal nerves (Figure 1B).4 The catheter is positioned within the main renal arteries and centred by an integrated low-pressure, saline-filled cooling balloon to achieve a circumferential ring of ablation. Treatment is delivered sequentially to the distal, mid and proximal main renal arteries, with each treatment lasting 7 seconds.
Alcohol-based RDN (e.g., Ablative Solutions) uses 3 retractable deep microneedles to deliver dehydrated alcohol into the perivascular space of the main and large accessory renal arteries (4–7 mm) causing nerve degeneration (Figure 1C).4
What is the evidence?
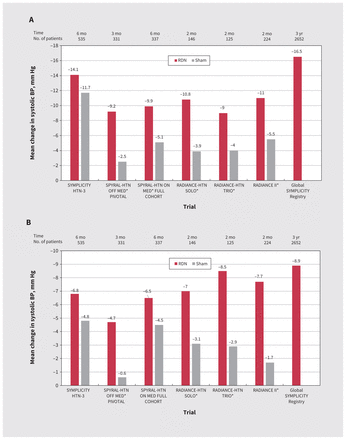
After the Symplicity HTN-3 trial, published in 2014, failed to show significant BP reductions with RDN (Figure 2), the field of RDN was almost abandoned.4 Since then, RDN technology, procedural techniques and results of additional clinical trials have improved. A series of randomized controlled trials using second-generation technology (Figure 2) showed that RDN lowered systolic BP by 5–10 mm Hg compared with baseline, providing the equivalent effect of 1–2 drugs, both in the presence and absence of antihypertensive medications.2,4,5
In the Spyral-HTN OFF MED trial, 331 patients (systolic BP 140–170 mm Hg) who were not using medications were randomized to receive radiofrequency RDN or a sham procedure. The 3-month change in 24-hour ambulatory systolic BP from baseline was lower with RDN than the sham procedure (−4.7 mm Hg v. −0.6 mm Hg, p = 0.0005).5 In the Spyral-HTN ON MED trial, 337 patients taking 1–3 antihypertensive medications were randomized to receive radiofrequency RDN or sham procedure.6 At 6 months, office systolic BP was reduced by RDN (–9.9 mm Hg v.–4.9 mm Hg, p = 0.001). However, the primary outcome of 24-hour ambulatory systolic BP at 6 months was not significantly different between the 2 groups, possibly owing to increased use of antihypertensive medications in the sham-control arm during follow-up and group differences in completion of ambulatory BP monitoring during the COVID-19 pandemic. No major RDN-related adverse events were observed by 6 months.
The ultrasound RDN system was evaluated in the absence (RADIANCE-HTN SOLO) and presence (RADIANCE-HTN TRIO) of antihypertensive medications (Figure 2).5 The larger RADIANCE II study randomized 224 patients (2:1) to ultrasound RDN or sham control in the absence of antihypertensive medications.7 The reduction in daytime ambulatory systolic BP at 2 months was greater among patients treated with ultrasound RDN (−7.9 [standard deviation 11.6] mm Hg) than sham controls (−1.8 [standard deviation 9.5] mm Hg), with a baseline-adjusted between-group difference of −6.3 mm Hg (95% confidence interval −9.3 to −3.2 mm Hg, p < 0.001). No major ultrasound RDN–related adverse events were observed by 6 months.
Alcohol-based RDN technology is under early-phase investigation for patients taking and not taking antihypertensive medications.4,5
Adequately powered, head-to-head comparisons of different types of RDN technology have not been performed. A persistent observation in RDN trials is the “always on” effect, with RDN lowering BP consistently throughout a 24-hour period.2,5,7 These findings have important implications for nonadherence and the shortcomings of drug regimens, such as cost, polypharmacy and adverse effects. Moreover, several studies have observed a reduction in pill count after RDN.2,5 Both registry data and sham-controlled trials indicate durable results to 36 months and beyond.5
A patient’s response to RDN is difficult to predict because of variability in baseline sympathetic hyperactivity or in renal nerve ablation; 10%–30% of patients do not respond to RDN, at least initially, and 20%–30% of patients have an above-average response to RDN. In the Global Symplicity Registry of 3077 patients receiving radiofrequency RDN, a greater BP response to RDN through 6 months — as measured by a 10% increase in time spent in therapeutic range (office systolic BP < 140 mm Hg), compared with time in therapeutic range before RDN — was associated with significant reductions in major adverse cardiac events (15%), cardiovascular death (11%), myocardial infarction (15%) and stroke (23%) 6–36 months after RDN.8 Currently, a simple, reproducible way to quantify sympathetically mediated hypertension before RDN and a reliable intraprocedural marker of successful RDN do not exist.4,7 Experience with repeat RDN procedures is limited.
Who may be eligible for RDN?
Patients with resistant hypertension — defined as BP higher than 140/90 mm Hg despite using 3 or more antihypertensive agents9 —are a subset of the larger population with uncontrolled hypertension. A major reason for lack of control is nonadherence (40%).2 Renal denervation may become an option in the foreseeable future for patients with uncontrolled hypertension for whom secondary causes of hypertension and white-coat hypertension have been ruled out and whose medications have been optimized to the extent possible.
Key considerations for treating with RDN include uncontrolled hypertension (office BP > 150/90 mm Hg despite guideline-based therapy, including health behaviour changes and 1–3 medications); elevated cardiovascular risk or endorgan damage (e.g., heart, kidney or brain damage, peripheral arterial disease); shared decision-making regarding risks, benefits, and circumstances affected by social determinants of health (e.g., low income, difficulty complying with complex medication regimens); and endorsement of candidacy by a hypertension expert and interventionalist. Exclusion criteria used in clinical trials were renal fibromuscular dysplasia, renal artery stenosis greater than 50%, previous renal artery stenting within the last 3 months, an estimated glomerular filtration rate less than 40–45 mL/min/1.73 m2, a single functioning kidney and previous kidney transplantation.
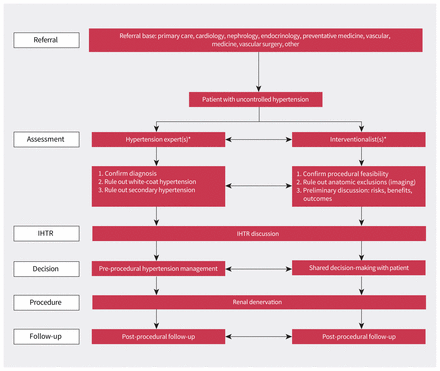
Our current preferred model of care for providing RDN involves an interventional hypertension centre of excellence comprising hypertension experts and experienced interventionalists (i.e., cardiologists, radiologists or vascular surgeons) who can select patients for RDN, ensure appropriate use of technology and provide high-quality care (Figure 3).2,5
Example of a multidisciplinary, team-based care pathway for renal denervation *The clinical specialty of the hypertension experts and interventionalists will vary from centre to centre depending on clinical interests and local expertise. The 2-sided arrows indicate interactions between team members at each level of care. Note: IHTR = interventional hypertension team rounds.
What are the harms?
Studies of RDN have not identified excess device- or procedure-related risks relative to sham-control patients (Table 1). The procedural risks are mostly those associated with femoral arterial catheterization (Table 1).5
Adverse effects observed in clinical trials of renal denervation
What are the resource implications?
The exact costs of RDN are currently unknown. Most procedures will require about 2 hours of time in an interventional suite, RDN catheters, a console and standard equipment employed for percutaneous vascular access and renal interventions (estimated cost $10 000–15 000 per procedure). Patients treated with RDN may be kept in hospital overnight for BP monitoring and adjustments to antihypertensive medications, as needed.
What can be expected in the future?
Renal denervation has regulatory approval in 60 countries, with approval in North America currently under consideration.10 Recently, a European consensus statement endorsed RDN as an adjunctive treatment for uncontrolled hypertension.5 In the future, more long-term efficacy and safety data will be available, and Canadian recommendations about the role of catheter-based therapies for hypertension are likely to be developed. In addition, more RDN systems will come to market. The techniques are likely to be refined; for example, radial artery access may reduce vascular access complications. Renal denervation will be investigated in populations that have sympathetic overdrive, including patients with chronic kidney disease, heart failure or atrial fibrillation.4
Renal denervation is an innovative catheter-based intervention for patients with uncontrolled hypertension. For patients with uncontrolled hypertension, medication intolerance or those unwilling or unable to commit to lifelong medication regimens, RDN may be an important treatment option to optimize care. The technology could be used as an adjunct to drug therapy and lifestyle modifications. If RDN gains approval in Canada, it has the potential to substantially improve the clinical management of patients with uncontrolled hypertension.
CMAJ invites contributions to Innovations, which highlights recent diagnostic and therapeutic advances. Novel uses of older treatments will also be considered. For publication, the benefits of the innovation, its availability and its limitations must be highlighted clearly, but briefly. Visual elements (images) are essential. Submit brief evidence-based articles (maximum 1000 words and 5 references) to http://mc.manuscriptcentral.com/cmaj or email andreas.laupacis{at}cmaj.ca to discuss ideas.
Footnotes
Competing interests: Sheldon Tobe reports payment from Medtronic Canada. Andrew Dueck reports payment for expert testimony, patents related to magnetic resonance imaging sequences for the evaluation of peripheral plaque. He holds the Maggisano chair in vascular surgery. Mina Madan reports participation on an advisory board for Medtronic Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work. Erez Marcusohn and Mina Madan drafted the manuscript. All of the authors revised it critically for intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/