Abstract
Background: Risk factors for severe outcomes of SARS-CoV-2 infection are not well established in children. We sought to describe pediatric hospital admissions associated with SARS-CoV-2 infection in Canada and identify risk factors for more severe disease.
Methods: We conducted a national prospective study using the infrastructure of the Canadian Paediatric Surveillance Program (CPSP). Cases involving children who were admitted to hospital with microbiologically confirmed SARS-CoV-2 infection were reported from Apr. 8 to Dec. 31 2020, through weekly online questionnaires distributed to the CPSP network of more than 2800 pediatricians. We categorized hospital admissions as related to COVID-19, incidental, or for social or infection control reasons and determined risk factors for disease severity in hospital.
Results: Among 264 hospital admissions involving children with SARS-CoV-2 infection during the 9-month study period, 150 (56.8%) admissions were related to COVID-19 and 100 (37.9%) were incidental infections (admissions for other reasons and found to be positive for SARS-CoV-2 on screening). Infants (37.3%) and adolescents (29.6%) represented most cases. Among hospital admissions related to COVID-19, 52 (34.7%) had critical disease, 42 (28.0%) of whom required any form of respiratory or hemodynamic support, and 59 (39.3%) had at least 1 underlying comorbidity. Children with obesity, chronic neurologic conditions or chronic lung disease other than asthma were more likely to have severe or critical COVID-19.
Interpretation: Among children who were admitted to hospital with SARS-CoV-2 infection in Canada during the early COVID-19 pandemic period, incidental SARS-CoV-2 infection was common. In children admitted with acute COVID-19, obesity and neurologic and respiratory comorbidities were associated with more severe disease.
As of Dec. 31, 2020, Canada had 581 427 confirmed cases of SARS-CoV-2 infection.1 Similar to other countries, most confirmed infections were in adults, in part because of initial testing policies that targeted older and at-risk populations, as well as prolonged societal containment measures to minimize children’s risk of exposure. Although fewer SARS-CoV-2 infections in children were reported relative to adults during Canada’s first waves of the pandemic,2 recent surges in pediatric cases across North America have challenged the notion that children are infected at a lower frequency than adults.3,4 However, the severity of infection in children appears to be substantially lower, with fewer overall hospital admissions reported and substantially lower mortality compared with adults.5,6
Although risk factors for more severe outcomes of COVID-19 have been well described in adults,7 similar risks are less well described in children.8 Experience with other viral respiratory infections, including influenza and respiratory syncytial virus, has shown that patient-level factors can increase risk for severe disease in children.9,10 Understanding populations at risk for severe disease is essential for developing evidence-informed testing strategies, recommendations around reducing exposure (including guidance informing in-person schooling) and potential prioritization of SARS-CoV-2 vaccines in children.
To date, few published data have characterized admissions to hospital with SARS-CoV-2 infection among children in Canada. We sought to describe pediatric hospital admissions associated with acute SARS-CoV-2 infection in Canada and identify risk factors for severe disease among children admitted to hospital.
Methods
The Canadian Paediatric Surveillance Program
The Canadian Paediatric Surveillance Program (CPSP), a joint project of the Canadian Paediatric Society and the Public Health Agency of Canada (PHAC), is a platform for public health surveillance that was designed to support national prospective pediatric studies.11 Using online case reporting, the CPSP gathers information on specific pediatric diseases through its network of more than 2800 pediatricians and pediatric subspecialists from across Canada, representing most of the providers of pediatric care in the country.11,12
In March 2020, at the onset of the COVID-19 pandemic, a CPSP COVID-19 study group was assembled, including representatives from academic and community pediatric centres from all regions of Canada. The study was designed to collect patient-level detail on 3 different groups: children admitted to hospital with acute SARS-CoV-2 infection (reported here), children with SARS-CoV-2 infection who were not admitted to hospital but who were younger than 1 year or had an underlying comorbidity and children with pediatric inflammatory multisystem syndrome associated with COVID-19 (launched in May 2020).13,14 This analysis includes all reported cases to Dec. 31, 2020. The study protocol, including case definitions and case report form, is available at https://www.cpsp.cps.ca/surveillance/study-etude/covid-19 (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content).
Beginning on Apr. 8, 2020, CPSP participants were asked to report all cases encountered in the previous 7 days involving children younger than 18 years of age who were admitted to hospital with acute, microbiologically confirmed SARS-CoV-2 infection, as well as all such cases that had not yet been reported. Participants who reported a case were asked to complete a case report form that included key demographic, epidemiologic, microbiologic and clinical data (including comorbidities).
Real-time case monitoring and data cleaning was conducted throughout the study period. When reporting a case, physicians could agree to further contact by the CPSP to clarify discrepant, missing or unclear data. If cases were reported in duplicate, records were compared and collapsed together using the most complete and accurate data from each record.
Case classification and severity
For all children with acute microbiologically confirmed SARS-CoV-2 infection and clinical data reported, we classified the cases into 1 of the following mutually exclusive categories: related to COVID-19, that is, a child admitted to hospital with clinical disease that was determined to be directly related to SARS-CoV-2 infection; incidental infection, that is, SARS-CoV-2 infection identified in a child admitted to hospital for an unrelated reason (e.g., fracture) and the infection did not alter the course of admission; or related to social or isolation purposes, that is, SARS-CoV-2 infection identified in an asymptomatic or mildly symptomatic child who required hospital admission for isolation or social purposes (e.g., caregiver[s] was admitted to hospital). To ensure accuracy of case classification, at least 2 study team members (O.D., C.M.H., S.K.M. or F.K.), reviewed all available information on the case report form, including all additional diagnoses and interventions reported during the hospital admission. Any discrepancies in the assignment of category of disease were resolved by follow-up with the physician or by consensus.
We categorized disease severity using a modified version of the Dong criteria that were adapted to local practice (Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content).15,16 We defined case severity as follows: asymptomatic, that is, patients reported to have no signs or symptoms, with radiologic findings normal or not conducted; mild disease, that is, symptomatic but without respiratory distress or abnormal radiology; moderate disease, that is, patients with lower respiratory tract disease, hematologic abnormalities or abnormal findings on radiologic examination but lacking other organ involvement and need for respiratory support; severe disease, that is, patients with respiratory distress or requiring supplemental oxygen; or critical disease, that is, patients admitted to the intensive care unit (ICU), requiring ventilation or experiencing clinical features of shock or other organ involvement. For analysis, we combined severe and critical categories as severe disease and mild and moderate categories as nonsevere disease. To categorize patients, details of reported radiologic findings were independently assessed by 2 investigators (O.D., C.M.H., S.K.M. or F.K.) and categorized as abnormal, abnormal but not related to SARS-CoV-2, nonspecific, or normal. Any discrepancy was resolved by consensus.
Statistical analysis
We analyzed demographic characteristics using frequencies, percentages, medians and interquartile ranges. We categorized age as infant (< 1 yr), preschool (1–5 yr), school age (6–12 yr) or adolescent (13–17 yr). Primary analyses included only cases admitted to hospital for COVID-19. We analyzed the following case characteristics: severity, comorbidities, signs and symptoms, concurrent infections, imaging and treatment(s). For age, sex, severity, and prematurity variables, we conducted the analyses as complete case and indicated the proportion of missing data in table footnotes. For all other variables, we imputed missing or unknown data to the null value and included these in the denominators. We assessed differences in age groups and severity categories using Fisher exact tests or χ2 tests. We used a significance threshold of α = 0.05 for all comparisons. We conducted the analyses using Stata (version 16.1). Because of CPSP policies, we reported frequencies between 1 and 4 as less than 5 and presented some outcomes as ranges. In some instances, results of subgroup analyses are not shown to prevent back-calculation of frequencies less than 5.
Ethical approval
The CPSP operates under legal authority derived from Section 4 of the Department of Health Act and Section 3 of the Public Health Agency of Canada Act. Ethics approval was obtained from the PHAC and Health Canada Research Ethics Board (REB 2020-002P), The Hospital for Sick Children (REB 1000070001), the Institutional Review Board of the Centre Hospitalier Universitaire Sainte-Justine (IRB MP-21-2021-2901) and at individual sites, as required by local policies. In Quebec, the study was conducted as a multicentre study, with clinical data collected by study co-investigators.
Results
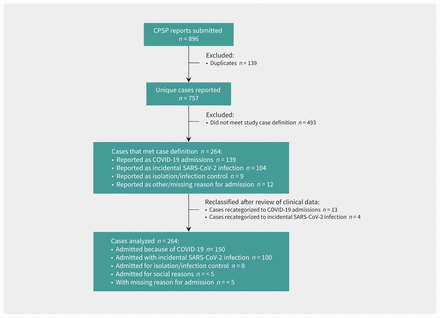
In total, 757 unique cases were reported to the CPSP with a date of diagnosis between Mar. 25 and Dec. 31, 2020, of which 264 met our study’s case definition (admitted to hospital with acute SARS-CoV-2 infection) and were included in the analysis (Figure 1). Cases were reported from all provinces; however, full data capture was not possible for Alberta because of delays in securing the appropriate data-transfer agreements. Our study reports on 80.0% of all hospital admissions of patients younger than 18 years of age in Canada excluding Alberta (246/308) as reported to PHAC during our study’s time period (unpublished data; source: case information received by PHAC from provinces and territories) and 68.6% in Canada with Alberta included. Most of these patients (all categories) were from Quebec (n = 114, 43.2%) and Ontario (n = 94, 35.6%) (Table 1), and were admitted to hospital either between April and June or between September and December 2020 (Figure 2).
Flow chart of cases reported to the Canadian Paediatric Surveillance Program (CPSP) COVID-19 study with a diagnosis of SARS-CoV-2 infection on or before Dec. 31, 2020.
Demographic characteristics of children admitted to hospital with SARS-CoV-2 infection before Jan. 1, 2021, in Canada
Hospital admissions for acute SARS-CoV-2 infections involving children, by calendar month and region of residence. Note: Number of new cases admitted in each month. Note: Western/Northern includes Alberta, British Columbia, Manitoba, Northwest Territories, Nunavut, Saskatchewan and Yukon. Atlantic includes Newfoundland and Labrador, New Brunswick, Nova Scotia and Prince Edward Island.
There were more reported SARS-CoV-2 infections among infants (< 1 yr; n = 97, 37.3%) and adolescents (13–17 yr; n = 77, 29.6%) than among preschool (1–5 yr; n = 44, 16.9%) or school-aged (6–12 yr; n = 42, 16.2%) children. Using population denominators from Statistics Canada,17 this represents a minimum incidence of hospital admission with SARS-CoV-2 infection of 26.0, 2.3, 1.4 and 3.8 per 100 000 population in the infant, preschool-and school-aged children, and adolescent age groups, respectively. A similar distribution was seen among those admitted to hospital because of COVID-19 (Appendix 3, Supplementary Table S1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content). We found that severity of SARS-CoV-2 infection did not differ significantly by sex, most population groups or region of residence; however, severe COVID-19 was more likely among children with comorbidities (p = 0.01) (Table 1).
Fewer than 5 children (< 1.9%) travelled out of their home province (within Canada or internationally) in the 12 weeks before symptom onset. There were 129 children (48.9%) with a close contact who was known to be SARS-CoV-2 positive in the 8 weeks before symptom onset. Close contacts were most often parents (n = 99, 76.7%), siblings (n = 28, 21.7%) and other relatives (n = 13, 10.1%). Twenty infections (7.6%) were nosocomially acquired (10 in long-term care facilities and 10 in hospitals). Seven children were reported to have a known contact at school or daycare, all of which occurred in September 2020 or later.
Categorization of cases is shown in Figure 1. Following investigator review, the cause of hospital admission remained unchanged from physician report in 93.6% of cases (Appendix 4, Supplementary Figure S1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content). Of the 264 cases, 150 (56.8%) were admitted because of COVID-19. The remainder (n = 114, 43.2%) included 100 children (87.7%) who were admitted to hospital for other reasons who were found to have incidental SARS-CoV-2 infection on screening, 8 children (7.0%) who were admitted for isolation or infection control after a positive result for a SARS-CoV-2 test and fewer than 5 children (< 4.4%) were admitted for social reasons. In fewer than 5 cases (< 1.9%), reason for admission was not specified and we could not categorize these because of insufficient data. Fifteen cases (5.7%) concurrently met the CPSP case definition for hospital admission with acute SARS-CoV-2 infection and the case definition for pediatric inflammatory multisystem syndrome.
The most common symptoms among the 150 children who were admitted to hospital with COVID-19 were fever (70.0%), vomiting (34.7%) and cough (34.4%) (Table 2). Common laboratory findings that were reported included lymphopenia (20.0%), anemia (14.0%) and neutropenia (13.3%). Coagulopathy occurred in 12.7% of these children, hepatitis in 11.3% and cytokine storm in 6.7%. Although fever was equally present in infants and older children (> 1 yr), older children were more likely to present with vomiting, anemia, pneumonia, hypotension, and subjective reports of headache and sore throat, whereas infants were more likely to present with runny nose, neutropenia or bronchiolitis. Appendix 5, Supplementary Table S2 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content) provides the clinical description of COVID-19 cases without those that also met the case definition of pediatric inflammatory multisystem syndrome.
Clinical symptoms at presentation and laboratory findings among children admitted to hospital because of COVID-19
Fifty-nine children (39.3%) had at least 1 comorbidity (Table 3). Chronic encephalopathy with severe neurodisability (11 ≤ n ≤ 14), obesity (11 ≤ n ≤ 14), asthma (10 ≤ n ≤ 13), chronic lung disease (excluding asthma) (10 ≤ n ≤ 13), epilepsy (10 ≤ n ≤ 13) and neurodevelopmental disorders (7 ≤ n ≤ 10) were the most common. Of children with epilepsy, 9 had a second neurologic or neurodevelopmental comorbidity reported, including 5 with cerebral palsy. There were no hospital admissions due to COVID-19 reported among children with inflammatory bowel disease, bone marrow transplantation or psychiatric conditions. Of children born prematurely (9 ≤ n ≤ 12), median chronological age was 0.9 years (interquartile range [IQR] 0.0–2.6 yr) and median gestational age at birth was 30 weeks (IQR 28–34 wk). Hospital admissions related to COVID-19 rather than not related to COVID-19 were more likely for children with asthma (6.7%–8.7% v. < 4.5%, p = 0.03) or metabolic disease (4.0% v. 0.0%, p = 0.04) and less likely for children born prematurely (6.5%–8.6% v. 18.7%, p = 0.03) or with a psychiatric condition (0% v. 4.5%, p = 0.01) (Appendix 6, Supplementary Table S3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210053/tab-related-content).
Associated factors and chronic comorbidities among children who were admitted to hospital because of COVID-19
Overall, 75 (50.0%) children were categorized as having non-severe disease and 75 (50.0%) as having severe disease (Table 4). The proportion of infants admitted to hospital with COVID-19 who met criteria for severe disease was less than for children older than 1 year (33.4% v. 62.8%; p = 0.001) (Table 4). Thirty-two cases (21.3%) were admitted to ICU (including < 5 to the neonatal ICU) and 9 required mechanical ventilation. Fewer than 5 children who were reported to CPSP died. Children who were admitted to hospital with severe disease (Table 3) were more likely to have an underlying comorbidity than children with nonsevere disease (52.0% v. 26.7%; p = 0.001). Those with severe disease were also more likely to have several underlying comorbidities (v. 0 or 1 comorbidity, n = 19, 25.3%) than those with nonsevere disease (n = 6, 8.0%; p = 0.005). Finally, children with severe disease were more likely to have epilepsy (v. none; 12.0% v. < 6.7%; p = 0.03), chronic encephalopathies with severe neurodisability (v. none; 13.3% v. < 6.7; p = 0.005), obesity (v. not obese; 13.3% v. < 6.7%; p = 0.04), or chronic lung disease (excluding asthma; v. none; 12.0% v. < 6.7%; p = 0.009). There was no difference in disease severity among children with immunosuppression compared with those who were immunocompetent (< 6.7% each; p = 1.00).
Illness severity, support provided and radiographic findings among children admitted to hospital because of COVID-19
About 1 in 4 patients received systemic corticosteroids and 7 (4.7%) received any of remdesivir, (hydroxy)chloroquine or other immunomodulators including biologics (Table 5). Eighty-one children (54.0%) received systemic antibiotics, of whom 40.7% were infants and 6.2% had sickle cell disease. No patient received SARS-CoV-2 convalescent plasma or monoclonal antibodies, and no child was reported to have been enrolled in a clinical trial for COVID-19 treatment. Children with a severe presentation were more likely to have received azithromycin, corticosteroids, immunoglobulin and anticoagulation (Table 5).
Treatments administered to children who were admitted to hospital because of COVID-19
Interpretation
We have described the clinical manifestations, characteristics, severity of disease and treatment of most children who were admitted to hospital with SARS-CoV-2 infection in Canada between Mar. 25 and Dec. 31, 2020. Although reporting to CPSP was voluntary, the engagement of pediatricians in Canada led to patient-level data being captured for about 70% of all children admitted to hospital with SARS-CoV-2 infection.
Our study shows that the clinical presentation and severity of disease caused by SARS-CoV-2 infection were different in children than in adults in the first part of the COVID-19 pandemic in Canada. Although children have recently been shown to have the highest seroprevalence of SARS-CoV-2 antibodies from infection among all age groups in Canada (3.3%), the relatively small number of pediatric hospital admissions highlights that children have less severe infection than adults even though they may be infected more frequently. 18 Many children with SARS-CoV-2 infection in Canada were likely undiagnosed because they were asymptomatic and were consistently underrepresented in proportions of positive test results reported across provincial jurisdictions.19,20 Our finding that nearly half (43.8%) of the children admitted to hospital with SARS-CoV-2 infection were admitted for other medical reasons, social reasons or infection control purposes reinforces this assumption. Among children admitted to hospital for COVID-19, half (50.0%) were categorized as having severe disease, 21.3% required admission to ICU and 13.3% required cardiac or respiratory support greater than low-flow oxygen
Our findings suggest a different pattern of severity compared with previous pediatric studies. A 2020 descriptive study involving 130 children in Italy who were admitted to hospital with COVID-19 found that 15.4% had severe or critical disease.21 Differences in classification of asymptomatic patients (incidental SARS-CoV-2 infection in our analysis) may explain the different findings. The rate of admissions to ICU in our study was higher than that reported in a 2020 systematic review22 but similar to those found in a large, multicentre prospective observational cohort study in the United Kingdom.23 Different clinical approaches across jurisdictions early in the pandemic, including tendencies to admit patients to ICU for close monitoring rather than for cardiac or respiratory support may explain differences in studies’ findings.
Similar to several published studies, deaths due to COVID-19 were rare in our study.24 Our findings align with findings from recent studies8,21,25 and a meta-analysis26 that suggested that children with certain comorbidities may be at higher risk of severe COVID-19.
Also similar to other studies, we found that most children admitted to hospital with acute COVID-19 required only supportive care, with only a few receiving targeted therapy for COVID-19 such as remdesivir, (hydroxy)chloroquine, biologic agents, anticoagulation, steroids or immunoglobulins.27 Although this may reflect the less severe nature of the disease in children, evolving evidence for these interventions in the pediatric age group may have also played a role. Of note, none of the children in this study were enrolled in any of the available clinical trials for children with COVID-19 in Canada, highlighting the challenges in conducting clinical trials in children and explaining, in part, the lack of an evidence base to guide pediatric care.
Our findings suggest a bimodal age distribution with peaks among infants and teenagers. Higher minimum incidence of hospital admissions for COVID-19 among infants, combined with observed smaller proportions with severe disease, may be explained by 2 factors. First, physicians seeing those patients may have applied greater caution among those patients (opting to admit to hospital for observation rather than discharging to home). Second, in many institutions, it is standard practice to admit to hospital any child younger than 6–12 weeks of age who presents with fever.24,28,29 These factors may also explain why our findings differ from other studies that reported that infants (< 1 yr) were at higher risk of severe disease.21,22 The higher proportion of cases among teenagers may reflect more severe disease in this age group. This group may also be at increased risk of acquiring infection compared with younger children.24,30
Although the number of children in this study with specific comorbidities was small, the absence of reported cases of children with any immune compromise (e.g., malignant disease, transplant recipients and those undergoing immunosuppressive therapy) may be cause for cautious optimism, but a relatively large number of children with neurologic conditions were reported. Further research on characteristics of this heterogeneous group is needed to determine whether certain populations (e.g., children with cerebral palsy or neurodevelopmental disability) are at increased risk from underlying medical complexity, unique exposure risks (e.g., multiple medical caregivers in home and school or residence in long-term care facilities) or a combination of both.31 As more data are reported, it will be important to track the clinical course of children with risk factors for severe disease from other respiratory viruses such as children born prematurely or those with asthma and other chronic respiratory conditions and any differences in severity associated with SARS-CoV-2 variants.
Limitations
Our study is limited by the voluntary reporting of the CPSP. Furthermore, complete case reporting was not possible from Alberta. As SARS-CoV-2 testing protocols varied by jurisdiction and throughout the study period and were limited in the early months of the pandemic to only symptomatic children, the total number of children admitted to hospital with incidental infection may be underestimated. We are unable to comment on the severity of identified underlying comorbidities and the numbers of reported cases with each comorbidity and risk factor precluded from conducting multivariable regression analyses to adjust for possible confounders. Although we described the distribution of cases by population group, we are unable to draw any conclusions because this variable was not reported by families. Finally, we conducted this study when substantial public health measures, including those related to physical distancing, were in place across the country and before the introduction of SARS-CoV-2 variants of concern to Canada. Changes to physical distancing practices and viral evolution may affect the epidemiology of COVID-19 in children. Nonetheless, our study was able to identify causes of admission and distinguish between incidental hospital admissions and those related to COVID-19 among children in Canada.
Conclusion
We found that the overall number of children admitted to hospital with SARS-CoV-2 infection in Canada was low, and nearly half of identified infections were incidental. As the pandemic continues to unfold, vigilance about potential changes in the epidemiology of SARS-CoV-2 infection in children is needed. A better understanding of overall disease severity in children, and at-risk groups of children, is important for plans related to future implementation of SARS-CoV-2 vaccines and prioritization in this age group.
Acknowledgements
The authors thank the pediatricians, pediatric subspecialists and health professionals who voluntarily respond to Canadian Paediatric Surveillance Program (CPSP) surveys. They also thank Dr. Jim Kellner for his guidance and support, as well as the members and leadership of the Paediatric Inpatient Research Network for cases reported and their dedication to the CPSP. The authors are grateful to the staff of the CPSP for their dedication, diligence and commitment to this study. The authors also thank members of the CPSP Scientific Steering Committee who serve as stewards of the program.
Footnotes
Competing interests: Krista Baerg reports funding from the Chronic Pain Network. She also served as Past President of the Community Paediatrics Section of the Canadian Paediatric Society and on the Board of Directors for Saskatchewan Pain Society, and has received royalties from Brush Education. Kevin Chan is Chair of the Acute Care Committee of the Canadian Paediatric Society. Elizabeth Donner is Chair of the Scientific Research Committee and a director of Epilepsy Canada. She is also a member of Partners Against Mortality in Epilepsy and the advisory boards of Cardiol, Pendopharm and Stoke Therapeutics. Catherine Farrell is Chair of the Scientific Steering Committee for the Canadian Paediatric Surveillance Program, former Chair of the Specialty Committee in Pediatrics of the Royal College of Physicians and Surgeons of Canada, former President of the Canadian Paediatric Society, and Chair of the Ethics Committee and a member of the Board of Directors of the Canadian Critical Care Society. She has received reimbursement for travel expenses from Canadian Paediatric Society and the Royal College of Physicians and Surgeons of Canada. She has also received an honorarium for a presentation at a continuing education conference from the Université de Sherbrooke. Sarah Forgie is the President of the Association of Medical Microbiology and Infectious Disease Canada. Fatima Kakkar has received honoraria for presentations given to the Association des Pédiatres du Québec. Thuy Mai Luu is the Director of the Canadian Neonatal Follow-Up Network. Charlotte Moore Hepburn is the Director of Children’s Mental Health of Ontario, and the Director of medical affairs for the Canadian Paediatric Society and the Canadian Paediatric Surveillance Program. Shaun Morris has received honouraria for lectures from GlaxoSmithKline. He was a member of an ad hoc advisory board for Pfizer Canada. Jesse Papenburg has received consultant fees from AbbVie, honouraria from AbbVie and Seegene, and he received respiratory virus testing materials from Seegene for his institution. He has participated in ad hoc advisory board meetings for AbbVie and is a voting member of the National Advisory Committee on Immunization. Rupeena Purewal is a consultant for Verity Pharmaceuticals. Manish Sadarangani is a member of the advisory board of Astra Zeneca. Marina Salvadori is an employee of the Public Health Agency of Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: The study was conceived by Olivier Drouin, Charlotte Moore Hepburn, Daniel Farrar, Fatima Kakkar and Shaun Morris. Olivier Drouin wrote the first draft of the manuscript. Analysis was conducted by Daniel Farrar. All of the authors contributed to data collection and interpretation, reviewed the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Elizabeth Donner has received a grant from the Ontario Brain Institute. Catherine Farrell has received a grant from Health Canada. Olivier Drouin, Jesse Papenburg, Thuy Mai Luu and Fatima Kakkar are supported by a Clinical Research Scholars Award from the Fonds de recherche du Québec – Santé. Shaun Morris has received an investigatorled grant from Pfizer Canada. Jesse Papenburg has received grants (paid to his institution) from MedImmune, Sanofi Pasteur and AbbVie. Manish Sadarangani has received grants (paid to his institution) from GlaxoSmithKline, Merck, Pfizer, Sanofi Pasteur, Seqirus, Symvivo and VBI Vaccines. He also receives financial support through salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research. Karina Top has received grants from GlaxoSmithKline. Financial support for the Canadian Paediatric Surveillance Program was received from the Public Health Agency of Canada (Morris) in support of this COVID-19 study.
Data sharing: De-identified data that underlie the results reported in this article (text, tables, figures and appendices) and that abide by the privacy rules of the Canadian Paediatric Surveillance Program and the Public Health Agency of Canada will be made available to investigators whose secondary data analysis study protocol has been approved by an independent research ethics board. Proposals should be directed to shaun. morris{at}sickkids.ca; to gain access, data requestors will need to sign a data access agreement.
- Accepted August 17, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/