Abstract
Background: Pharmacogenomic testing to identify variations in genes that influence metabolism of antidepressant medications can enhance efficacy and reduce adverse effects of pharmacotherapy for major depressive disorder. We sought to establish the cost-effectiveness of implementing pharmacogenomic testing to guide prescription of antidepressants.
Methods: We developed a discrete-time microsimulation model of care pathways for major depressive disorder in British Columbia, Canada, to evaluate the effectiveness and cost-effectiveness of pharmacogenomic testing from the public payer’s perspective over 20 years. The model included unique patient characteristics (e.g., metabolizer phenotypes) and used estimates derived from systematic reviews, analyses of administrative data (2015–2020) and expert judgment. We estimated incremental costs, life-years and quality-adjusted life-years (QALYs) for a representative cohort of patients with major depressive disorder in BC.
Results: Pharmacogenomic testing, if implemented in BC for adult patients with moderate–severe major depressive disorder, was predicted to save the health system $956 million ($4926 per patient) and bring health gains of 0.064 life-years and 0.381 QALYs per patient (12 436 life-years and 74 023 QALYs overall over 20 yr). These savings were mainly driven by slowing or avoiding the transition to refractory (treatment-resistant) depression. Pharmacogenomic-guided care was associated with 37% fewer patients with refractory depression over 20 years. Sensitivity analyses estimated that costs of pharmacogenomic testing would be offset within about 2 years of implementation.
Interpretation: Pharmacogenomic testing to guide antidepressant use was estimated to yield population health gains while substantially reducing health system costs. These findings suggest that pharmacogenomic testing offers health systems an opportunity for a major value-promoting investment.
Pharmacogenomic testing offers the possibility of enhanced efficacy and fewer adverse effects from more appropriately targeted drug therapies. One clinical area for which pharmacogenomics could have particular promise is major depressive disorder (MDD), given that patients with MDD often receive several trials of pharmacological treatment. The World Health Organization predicted that by 2030 depression will be the leading cause of disability worldwide.1 The lifetime prevalence of depression is 11% in Canada2 and the global prevalence increased by 28% in 2020, during the COVID-19 pandemic.3 People with MDD have higher rates of mortality4 and comorbidities,5 and a lower quality of life.6 Depression is costly for patients and society. In Canada, MDD costs $14 billion annually, largely driven by decreased productivity and workforce participation.7 Patients face personal financial burdens associated with depression and its treatment, including lost wages and out-of-pocket costs.8 Therefore, improving care for patients with MDD offers the potential for substantial, widespread impact.
More than 35 antidepressant medications are available in Canada; however, only 40%–60% of patients respond to the antidepressant initially prescribed, and roughly 27% report adverse effects.9–11 A large proportion of patients have partial or no response to several trials of treatment (i.e., treatment-resistant depression or refractory MDD).12,13 The lengthy trial-and-error process of selecting the appropriate medication may contribute to high nonadherence rates and, subsequently, to poorer health and long-term prognosis.14 Evidence suggests that response to antidepressants partly reflects variation in genes that influence medication metabolism. About 42% of the variation in the clinical response to antidepressant treatment may be attributable to genetic causes.15 Pharmacogenomic testing, therefore, is theoretically compelling; using a blood, saliva or buccal swab sample, the test identifies genetic variants involved in drug metabolism and response, which can guide prescribing. Meta-analyses suggest that pharmacogenomic testing can positively affect response and remission when used to guide treatment for MDD.16,17
Several previous cost-effectiveness analyses have evaluated the use of pharmacogenomic testing in MDD.18–22 However, many did not model the individual genetic profile of patients (an important factor in precision medicine), did not consider individual drugs, were funded by test manufacturers and used relatively short time horizons. We sought to evaluate the effectiveness and cost-effectiveness, including the long-term costs and benefits, of implementing pharmacogenomic testing to guide antidepressant care for MDD in British Columbia, Canada.
Methods
Model design and inputs
We developed a discrete-time microsimulation model in close collaboration with patient partners,23 clinicians and other stakeholders to facilitate the evaluation of the effectiveness and cost-effectiveness of pharmacogenomics for adult patients with newly diagnosed (incident) and prevalent MDD in BC. This simulation model of major depression (SiMMDep) is a discrete-time microsimulation model, built in C++ with an interface in R, using the Rcpp package.24,25
The SiMMDep model includes 8 interconnected modules for entry cohort, demographics, condition progression, treatment, adverse events, hospital admission, cost and quality-adjusted life-years (QALYs) and death. It has more than 1500 input parameters populated by estimates derived from a systematic review of randomized trials of pharmacogenomic testing efficacy in MDD treatment,16 original data analyses of BC administrative data,26–31 targeted searches and clinical expert panel judgments (when data were not available). Our panel of clinical experts comprised 1 family physician (M.P.), 1 psychiatrist (C.S.) and 1 psychologist (D.E.). Patient partners (L.R. and G.L.) contributed substantially to model design by verifying modelling assumptions, pointing out model limitations and suggesting areas for future research.23
We analyzed BC administrative data for the years 2015–2020 for people aged 19–99 years in BC who satisfied the criteria for depression.32 We linked several data sets, including the Medical Service Plan (MSP),26 Discharge Abstract Database,27 MSP Consolidation File,28 Vital Statistics Deaths,29 PharmaNet30 and National Ambulatory Care Reporting System.31 The model design and input analyses are detailed in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221785/tab-related-content.
We designed the model structure to represent the MDD clinical pathway using a combination of the Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline9 and advice from clinical experts (M.P., C.S., D.E.) and patient partners (L.R. and G.L.). The pathway includes 40 different antidepressants (pharmacotherapies) and other treatment options, such as electroconvulsive therapy (ECT) and individual psychotherapy. Medications included in the model were indicated for MDD treatment in the CANMAT guideline9 and publicly covered in part or in full by BC Pharmacare (Appendix 1, Appendix A, Table A4).
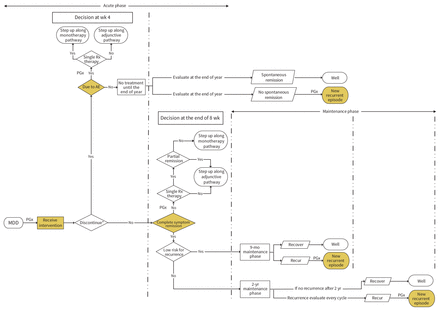
The SiMMDep model follows simulated patients individually over time as they move between health states (i.e., well, MDD and death). The duration of each cycle in the model is 1 week. All patients enter the model in the MDD state. After receiving treatment, patients may have an event, such as stopping treatment, remission (full or partial) or recurrence (Figure 1). In the event of full remission, the patient moves to the well health state. If the patient stops treatment because of an adverse event, has partial symptom remission or has recurrence during the maintenance phase, the patient stays in the MDD state and starts a new treatment trial.
The generic flow of patients with episodic major depressive disorder (MDD) in a treatment trial. Dashed lines separate the time points at which a pharmacotherapy treatment trial is evaluated for treatment discontinuation (wk 4), symptom remission or response (wk 12) and depression recurrence (9 mo later, at wk 52, and 2 yr later, at wk 116). Points along the pathway where the pharmacogenomic testing can occur are indicated (PGx). Patients with prevalent MDD would receive pharmacogenomic testing before any prescription, and patients with a new diagnosis of MDD would receive pharmacogenomic testing after 1 unsuccessful medication trial. In both instances, only patients with moderate-to-severe MDD would receive pharmacogenomic testing. Note: AE = adverse effect (e.g., nausea, weight gain), Rx = medical prescriptions. *Treatment may be stopped because of adverse effects or other reasons (e.g., feeling better, experiencing other serious diseases).
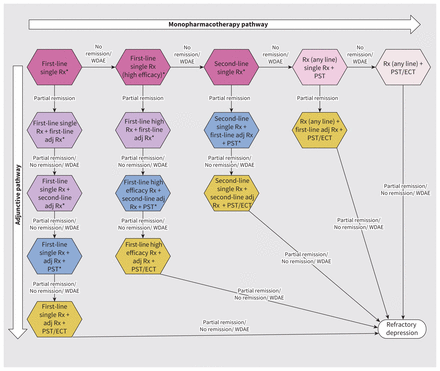
According to the MDD clinical pathway, patients in the pharmacogenomic-guided and current standard-of-care arms can have up to 5 treatment trials of any of the 6 different treatment options, which are progessively more intense, involving pharmacotherapy alone (single or double pharmacotherapy) or pharmacotherapy with either psychotherapy or ECT (Figure 2). The SiMMDep model was developed from the public payer perspective. Pharmacotherapy is the first treatment option available through public insurance in BC; psychotherapy is covered only after several unsuccessful medication trials, and ECT is the sole neurostimulation treatment that is publicly available. Patients who do not achieve full remission even after going through the most intensive treatment option remain in the MDD state are assumed to have refractory depression, also referred to in the clinical literature as stage V treatment-resistant depression.33 This ensured that patients had the opportunity to try several treatment options before their depression was labelled as refractory.
Clinical pathway of patients with major depressive disorder (MDD) under the current standard of care in British Columbia. The clinical pathway includes 6 different treatment options, represented by different colours on the graph. We assumed that patients with newly diagnosed MDD started from the beginning of the pathway. We assigned patients with prevalent MDD to one of the 9 starting points, represented by asterisks based on the prescription patterns from the BC administrative data. Note: adj = adjunctive, ECT = electroconvulsive therapy, PST = psychotherapy, Rx = medical prescription, WDAE = withdrawal due to adverse effect.
In the base-case analysis, pharmacogenomic testing was offered at different points along the clinical pathway, depending on the severity of the current episode and previous MDD history (Table 1). The distribution of MDD severity came from sources noted in Appendix 1, Table A1. Given the tendency for randomized controlled trials to recruit patients with moderate-to-severe MDD,16 the model restricted patients with mild MDD from receiving pharmacogenomic testing unless their condition recurred as a moderate or severe episode. Although pharmacogenomic testing is not part of the current standard of care in BC, patients can pay directly for these tests; however, they are costly36 and coverage from Canadian insurers is recent and variable.37 Therefore, we did not assume any use of pharmacogenomic tests in the current standard-of-care arm.
Delivery of pharmacogenomic testing in the base-case analysis for patients with prevalent and newly diagnosed major depressive disorder (MDD), by MDD severity
Pharmacogenomic testing for antidepressant medications identifies the variants in CYP2C19 and CYP2D6 genotypes — the 2 genes known to contribute to antidepressant efficacy and adverse effects38,39 — that are used to predict metabolizer phenotypes, namely poor metabolizers, intermediate metabolizers, normal metabolizers, rapid metabolizers (CYP2C19 only) and ultrarapid metabolizers.40 These phenotypes, in turn, may affect antidepressant efficacy or tolerance. For example, poor metabolizers may achieve higher serum concentrations of the medication, leading to increased adverse effects, need for a lower dose or a different antidepressant being preferred, while ultrarapid metabolizers may achieve lower serum concentrations, leading to reduced efficacy or need for a higher dose. The SiMMDep model uses the results of pharmacogenomic testing to assign medications to the patient according to their CYP2D6 and CYP2C19 metabolizer phenotypes41,42 and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines. 38–40 The CPIC guidelines are evidence-based recommendations for the use of pharmacogenomic testing in clinical practice, which include suggestions for dosage modifications or alternative medications based on the patient’s genetic profile. We also used the Sequence2Script tool to build a list of eligible medications available for each patient, according to these same metabolizer phenotypes (Appendix 1, Tables A5 and A6).43 For each pharmacological treatment, the model selects a medication based on CANMAT 2016 guideline, 9 the patient’s antidepressant history (recorded in the model) and the patient’s pharmacogenomic test result (if available). As such, SiMMDep assigned medications in line with the distribution of antidepressants currently prescribed in BC (Appendix 1, Table A4). Further, the model excludes any medications for that individual patient if they have previously caused an adverse event, did not result in full remission or are not appropriate based on the patient’s metabolizer phenotype. Considering every potential combination of CYP2D6 and CYP2C19 metabolizer types, the model eliminates all contraindicated medications for both metabolizer phenotypes in isolation, and generates a list of available treatment options. The model then adjusts the medication distribution based on the antidepressants that can still be prescribed and selects one based on the adjusted distribution. Next, the model adjusts the probability of stopping treatment (because of adverse effects or other reasons) and remission (partial and full) to reflect the test efficacy (based on the meta-analysis conducted by our team16). We have not assumed any additional remission benefit for psychotherapy or ECT based on phenotype information, because no evidence has suggested an additional benefit from pharmacogenomic testing. The meta-analysis found that the use of pharmacogenomics was associated with patients being 1.46 times more likely to achieve full remission and 1.20 times more likely to achieve partial remission. In addition, it showed that patients who had undergone pharmacogenomic testing were 11% less likely to stop pharmacotherapy and 57% less likely to stop because of adverse effects. The incidence and type of adverse effects vary by medication, and so we used this pooled probability of stopping pharmacotherapy to capture the impact of pharmacogenomic testing on adverse effects. We used the average cost of the pharmacogenomic tests available in Canada ($738)36 and then explored the price range in the sensitivity analyses.
Our analyses provided estimates of the incremental cost, QALYs and life-years for care pathways with and without pharmacogenomic testing for the entire 2021 cohort of BC adults (aged 19–99 yr) with MDD who were eligible for pharmacological treatment, over a 20-year time horizon. This time horizon allowed us to capture the longer-term costs and consequences associated with the implementation of pharmacogenomics. The cost estimates are from a public payer perspective and reported in 2020 Canadian dollars. We discounted costs and benefits at 1.5% annually, in line with the Canadian guideline.44
Sensitivity analyses
We carried out model validation exercises to check the face validity, internal validity and cross-validity (Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221785/tab-related-content). We performed sensitivity analyses to assess the robustness of results to variations in key model parameters and assumptions. We varied parameter values using 95% confidence intervals (CIs) when available or with ranges 50% above and below the mean values (Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221785/tab-related-content). We repeated the primary analysis using a lifetime time horizon. We also employed a probabilistic analysis to incorporate second-order uncertainty into the model. First, we identified a set of primary model input parameters, which were subsequently sampled from their corresponding distributions (Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221785/tab-related-content). We selected these input parameters in consultation with various stakeholders, including policy-makers. We ran the model 10 000 times (using the full sample in each iteration) to provide a sufficient representation of the model’s stochastic nature and to investigate the spectrum of the selected input parameters.
Ethics approval
This study was approved by the University of British Columbia Clinical Research Ethics Board (#H20-02362).
Results
Base-case analysis
The 2021 cohort of BC adults (aged 19–99 yr) with MDD included 194 149 people who were eligible for pharmacological treatment (mean age 45.6 yr). The base-case analysis showed that pharmacogenomic testing for patients with moderate-to-severe MDD resulted in higher survival, greater QALYs and overall cost-savings for the BC health care system. Over a 20-year time horizon, pharmacogenomic testing was a dominant strategy, with gains of 0.064 life-years and 0.381 QALYs per patient, or 12 436 life-years and 74 023 QALYs for all of BC, compared with care pathways without access to pharmacogenomic testing (Table 2).
Base-case results of pharmacogenomic-guided treatment, compared with current standard of care, for major depressive disorder (MDD) in British Columbia over a 20-year time horizon*
In the model, the introduction of pharmacogenomic testing would reduce the frequency with which MDD became refractory to treatment. Through a combination of higher remission rates and lower discontinuation rates, pharmacogenomic testing resulted in 23 216 (37%) fewer patients developing refractory depression; this was the main driver of cost savings. The use of pharmacogenomics was also associated with decreased use of more resource-intensive treatment options, such as psychotherapy and ECT (by 22% and 28%, respectively; Table 3). The availability of pharmacogenomic testing resulted in patients spending 15% more time in the well state and 18% less time in the MDD state (recurrent episodes or refractory depression). Reducing time spent in the MDD state resulted in 1869 fewer deaths and 21 346 fewer all-cause hospital admissions over 20 years.
Clinical results and resource use with pharmacogenomic-guided treatment compared with current standard of care, for major depressive disorder in British Columbia over a 20-year time horizon
From a cost perspective, the added expenditure for pharmacogenomic testing ($121 million) and increased episodic care ($524 million) were offset by a decrease in the cost of refractory MDD care (−$1.6 billion). Taken together, the overall cost savings from a public payer perspective was estimated to be $956 million over the 20-year time horizon, or a cost savings of $4926 per patient.
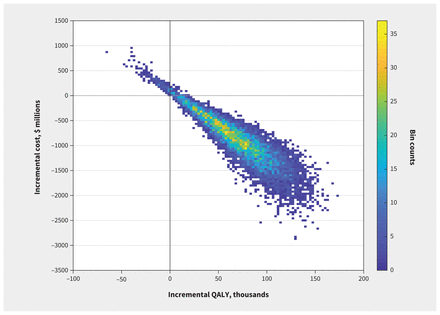
The pharmacogenomic treatment strategy dominated the current standard of care for most (96.79%) simulations (Figure 3). At a willingness-to-pay threshold of $50 000 per QALY, there is a 99.75% chance that the pharmacogenomic-guided treatment is cost-effective over 20 years (Appendix 4, Figure D1).
Cost-effectiveness density plot of 10 000 Monte Carlo iterations (20-yr time horizon and 1.5% discount rate). The darker shades of blue represent low density, while the lighter shades of yellow represent high density. Most (96.79%) simulations fall in the southeast quadrant (bottom-right) of the cost-effectiveness plane, indicating that the pharmacogenomic-guided strategy saves costs and increases quality-adjusted life-years (QALYs).
Sensitivity analyses
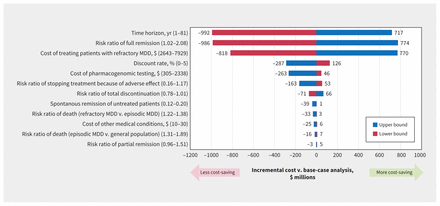
The sensitivity analyses showed that time horizon, pharmacogenomic test effectiveness (measured as full remission rate) and the cost of refractory depression were the parameters that had the most influece on cost-effectiveness (Figure 4 and Appendix 3).
Tornado diagram of the incremental cost, compared with the base-case analysis. We conducted a 1-way sensitivity analysis for all listed parameters. Note: MDD = major depressive disorder.
The selection of the time horizon for the analysis was an important driver of results. When considering different time horizons, our results indicated that the upfront investment in pharmacogenomic testing is typically offset after 2 years through lower direct medical costs, and is cost-saving from that point forward. As expected, with a lifetime time horizon, the strategy of pharmacogenomic testing became even more dominant, with much greater QALYs and cost savings.
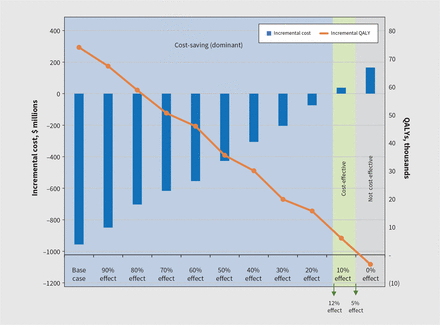
Concerning the effectiveness of pharmacogenomic test, we undertook a multivariate sensitivity analysis given the multiple effectiveness parameters, namely full remission, partial remission, total discontinuation and discontinuation because of adverse effects. Our analyses indicated, as would be expected, that lower effectiveness brings less cost savings, with such savings (over 20 yr) being almost zero when we assume effectiveness is only 12% of what we observed in our meta-analysis (Figure 5).16
Cost-effectiveness of pharmacogenomic testing as its clinical efficacy decreases. The clinical efficacy of pharmacogenomic testing in the base-case analysis is based on a combination of 4 different clinical parameters extracted from our systematic review, namely full remission (risk ratio [RR] 1.46), partial remission (RR 1.20), total discontinuation (RR 0.89) and discontinuation due to adverse effect (RR 0.43).16 In this multivariant sensitivity analysis, we varied these parameters at the same rate (closer to 1, which represents no difference with the current standard of care arm) to estimate the impact on incremental costs and quality-adjusted life-years (QALYs). For example, the new values of the RRs at 75% effect are 1.33 for full remission, 1.15 for partial remission, 0.91 for total discontinuation and 0.53 discontinuation because of adverse effects.
Interpretation
Our findings indicate that pharmacogenomic testing for patients with moderate-to-severe MDD both reduces costs from a public payer perspective and increases life-years and QALYs over a 20-year time horizon. The larger gain in QALYs compared with gains in life-years reflects the fact that depression affects the quality of life more substantially than survival. The effectiveness of pharmacogenomic testing has been established elsewhere, 16,17 with benefits seen in terms of enhanced remission rates and reduced levels of adverse effects for patients in their use of antidepressants. Our economic analysis shows the farranging impact that pharmacogenomics could have by slowing or avoiding the transition to refractory depression and avoidance of some interventions with high resource intensity, such as psychotherapy and ECT. These findings not only point to major cost savings for health care systems, but also to alleviation of some of the current human resource challenges in health care. Through the adoption of pharmacogenomics, the opportunity likely exists to reallocate limited resources to other parts of the health system and deliver further benefits to other patient groups.
Earlier economic analyses of pharmacogenomic testing for psychiatric medications also found favourable results, with cost savings predicted.18–21 However, most of the previous economic work in this field was funded by pharmacogenomic test manufacturers and was not conducted in a Canadian setting. In contrast, a recent Canadian, publicly funded health technology assessment of pharmacogenomic testing for MDD concluded that the evidence for the clinical effectiveness of pharmacogenomic testing in this setting remained uncertain and with low confidence in the effect.22 Unlike this previous study, our effectiveness review was restricted to randomized controlled trials and evaluated pharmacogenomic tests comprehensively as a class, rather than restricting analyses to a single test made by 1 specific supplier (i.e., excluding some evidence, resulting in a smaller sample size with less power). The economic analysis done as part of that health technology assessment used a 1-year time horizon (in contrast to our 20-yr time horizon) and assumed the most expensive pharmacogenomic test currently available in Canada (i.e., $2500, as opposed to our use of the average price of $738).22 Of note, in their sensitivity analyses, they showed that the use of pharmacogenomic tests could be cost-saving if the price was equivalent to several commercially available tests in Canada (i.e., $595).36
A particular strength of our research is that the results come from a new Canadian MDD model that includes a microsimulation modelling approach to incorporate unique characteristics of patients, such as geographic ancestry and metabolizer phenotypes. This allows the analysis to have direct relevance to clinical populations seen in Canadian jurisdictions. A unique element of our model is that it is drug-specific, thereby incorporating most prescribed antidepressants in Canada. As a result, it considers the impact of pharmacogenomic testing not only on the risk of remission and of discontinuation, but also on the medication selection process. Therefore, the model can predict prescription volumes of each medication in different policy strategies. This functionality is especially important to forecast procurement needs for those concerned with medication supply. The 20-year time horizon is a major strength of this model as it ensures that downstream impacts are captured, especially in the uptake of subsequent and more resource-intense treatments. The flexible modelling approach taken in our work allows for application to other health systems with appropriate tailoring.
Limitations
The SiMMDep model does not simulate post-refractory depression and therefore may underestimate the true benefit of pharmacogenomic testing. In the absence of available evidence, we assumed the average weekly costs of all comorbidities and treatments for such patients, an average health score and an average mortality rate, thus conservatively estimating the true costs for patients with complex needs. As with all modelling exercises, we were subject to data limitations, such as the lack of data on the direct impact of pharmacogenomic testing on the probability of recurrence. As a consequence, we applied the same probability of recurrence after remission for all patients in the model. Once again, this is a conservative approach; if testing produces longer-lasting effects on recurrence, these benefits were not captured by our analyses, and if built in, pharmacogenomic-guided treatment would have been even more cost-saving.
Our work has focused on establishing the effectiveness and cost-effectiveness of pharmacogenomic testing for MDD in a Canadian context. As a result, we have not modelled the implementation process and the transition that would be required to operate pharmacogenomic testing in Canadian health systems. For example, we did not incorporate the costs of establishing the infrastructure to implement pharmacogenomic testing to guide MDD treatment or to store the results. However, if we assume that implementation process costs might add $1600 per patient (the difference between the cost of the most expensive pharmacogenomic test available minus the average price of tests available in Canada), pharmacogenomic-guided treatment would still be cost-saving.
SiMMDep models publicly funded treatment pathways in BC, and so the results may not apply to populations with access to private-pay treatments, such as those with private insurance. Moreover, our results are specific to BC and are not necessarily generalizable to other parts of Canada, given jurisdictional variations in geographic ancestry, prescribing patterns and clinical pathways for MDD.
Conclusion
Major depressive disorder is common, recurrent and a large driver of health care costs and health burdens in Canada and other jurisdictions that is increasing, especially since the COVID-19 pandemic. Interventions that might improve remission rates and reduce the number of cases of refractory depression, in particular, are needed to improve the quality of life for patients, and reduce the economic burden of MDD on already strained health care systems.
The SiMMDep model represents an analytic infrastructure for clinical care of MDD in Canada, focused on guiding improvements in the effectiveness, efficiency and equity of care pathways for MDD. The analyses presented here point toward pharmacogenomic testing, focused on adults with moderate-to-severe MDD, offering the opportunity for a major value-promoting investment by health systems. Pharmacogenomic testing, focused in this way, has the potential both to reduce costs and improve health outcomes.
Acknowledgements
The authors thank the larger PGx4Dep team for their contributions to this publication. They specifically thank Dr. Mohsen Sadatsafavi for his support and contribution to the development of the analytical infrastructure and Dr. Chad Bousman for providing valuable input to incorporate the individual genetic profile of patients into the model. The authors offer gratitude to the Coast Salish Peoples, including the xʷməθkwəy̓ əm (Musqueam), Skwxwú7mesh (Squamish) and Səl̓ilwətaʔ/Selilwitulh (Tsleil-Waututh) Nations, on whose traditional, unceded and ancestral territory the authors have the privilege of working.
Footnotes
Competing interests: Christian Schuetz reports research funding from Provincial Health Service Authority, Health Canada, Canadian Institutes of Health Research, Canadian Centre on Substance Use and Addiction (CCSA) and Clairvoyant; consulting fees from CCSA; and travel support from Suchtmedizin. He is chair of the addiction and psychosis committee of the International Society of Addiction Medicine; research lead, Mental Health and Addiction with the Provincial Health Service Authority; division head, Substance Use and Concurrent Disorders with the University of British Columbia; and sits on the scientific committee of the World Association on Dual Disorders. Jehannine Austin is vice president of the International Society for Psychiatric Genetics and associate editor with the Journal of Genetic Counseling. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Shahzad Ghanbarian, Jehannine Austin and Stirling Bryan contributed to the conception and design of the work. All of the authors contributed to data acquisition, analysis and interpretation. Shahzad Ghanbarian drafted the manuscript. Gavin Wong, Louisa Edwards, Jehannine Austin and Stirling Bryan revised it critically for important intellectual content. All of the authors reviewed the final version, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by Genome BC, Genome Canada and Michael Smith Health Research British Columbia.
Data sharing: Access to data provided by the Data Steward(s) is subject to approval, but can be requested for research projects through the Data Steward(s) or their designated service providers.
Disclaimer: All inferences, opinions and conclusions drawn in this publication are those of the author(s) and do not reflect the opinions or policies of the Data Steward(s).
- Accepted September 18, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/












Podcast