This guidance for policy proposes a core set of elements for documents used to obtain participant consent for human genomics research in Canada.
The core set of elements comprises the essential components needed to ensure appropriate engagement of patients and other participants in genomics research and in the Canadian Human Genome Library (to be launched in 2023).
The benefits of a core set of consent elements include rationalization of approval for human genomics research projects by research ethics boards, and increased sharing of genomic and associated health information data across the country.
Use of a standardized set of consent elements can support the development of the federated Canadian Human Genome Library, in which advanced machine learning methods can be applied to determine which genetic factors contribute to health and disease for those living in Canada.
Human genomics — the study of the entirety of a person’s or population’s genes — is increasingly being integrated into research and rapidly incorporated into clinical care, including into health records.1,2 If most genomic data obtained in Canada could be accessed and analyzed collectively, the level of understanding of the role of genomics in determining health and predisposition to disease among Canadians would increase substantially. A pan-Canadian Human Genome Library (CHGL), a central node for the federated sharing of locally held genomic and associated health and medical information, is to be launched in 2023. The CHGL will empower the application of machine learning to large-scale genomic data, such that genetic factors that contribute to health and disease can be more accurately determined for people living in Canada.
The lack of data sharing among research groups is a widespread issue in research;3,4 concern about this resulted in the development of the findable, accessible, interoperable and reusable (FAIR) data principles,5 which define beneficial data stewardship practices. The establishment of the CHGL will enable these principles to be applied to genomics research in Canada, and simultaneously accelerate the development of critical tools to maximize the analysis of human genomes. Additionally, the CHGL will provide a single contact point to facilitate Canada’s participation in large-scale international research projects.
A harmonized set of tools and procedures is required to maximize the utility of the CHGL and enable genomic data sharing in Canada locally and nationally, including that of additional types of molecular genetic information that may be detectable in the future, such as epigenetic profiles, mitochondrial DNA profiles or transcriptional profiles. Developing standard approaches to data collection (where appropriate) can reduce bureaucratic burdens by making the minimum requirements explicit, and can facilitate development of common material for consultation, education and training. One important tool is a standardized core set of consent elements for human genomics research in Canada.
The purpose of this guidance for policy is to present a core set of elements for participant consent documents to be used in local human genome–based research projects across Canada and to support the development of the national CHGL. These core elements can be also used as a research ethics tool when evaluating human genome–based research projects.
Scope
This guidance is intended for researchers who engage in, and the research ethics boards that evaluate, human genomics research in Canada. Although the primary intent of this guidance is that the proposed core set of elements be used for consenting participants into the CHGL, it may also be used for other research projects, to facilitate sharing of genomic data.
Although this guidance includes the core elements that should be considered when evaluating human genomics research, additional considerations beyond those captured in the proposed set may be required based on local policy or law. The standard produced here is not intended to be mandatory or static. Individual research ethics boards are expected to make changes and adaptations, as necessary. Additionally, researchers are expected to enrich the consent document by including components specifically related to their projects.
The core elements are not meant to replace (but can be used to guide) information given to participants in research studies, as each project will require wording specific to the population(s) being studied. Additionally, they are not meant to be used in a clinical setting, other than for consenting patients for research, although aspects within these elements may be helpful to those using genomics in health care.
Recommendations
The minimal core set of genomic consent elements we developed will allow researchers to collect human genome data in a uniform manner that clearly explains current and future unspecified use to participants. The elements address topics such as research data; international sharing; commercial and future research use; storage, including location and duration; controlled access; reidentification and recontact of participants; and assent of minor participants (Table 1). The examples and explanations for the core set of consent elements are tailored for the CHGL; wording may vary depending on requirements of other research.
Recommendations for core elements for participant consent documents used in human genomics research in Canada
These topics emerged as key considerations during literature and document reviews and the consultation process described in the Methods section of this paper, as well as from established Canadian compliance requirements set out in policies such as the second Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2).6
Data collected
The consent documents should include a description of the research data that will be collected; for example, data from whole genome or exome sequencing, or ongoing collection of clinical data from medical records, administrative data or cohort data (which may include information on tests done and treatments received).7–9 The document should allow participants to withdraw data at any time and should note that it may not be possible to withdraw data already accessed and in use by a particular research study.
Use of data
Participants should be informed that the genetic and clinical data will be shared internationally among researchers who may be affiliated with a range of institution types, including industry.
Consent documents should make clear how the findings will be used for future research (for example, on a range of health outcomes) or that the data may be used commercially for the development of new diagnostic tests, new drugs or other commercial products, for which participants will not receive any share of profits or compensation. Additionally, participants should be made aware that their data could be compiled with that of others to aid in determining what aspects of a genome may be useful in predicting what predisposes people to disease and keeps them healthy. Deidentified data may be used in research publications and other forms of knowledge dissemination.5,9–11
Privacy and storage
To address issues of privacy, potential participants should be reassured that sharing of genetic and clinical data will be available only through a controlled access mechanism. There is a low risk that participants could be reidentified, but reidentification may be possible, given unknown technology advances in the future. Data will be stored indefinitely on servers located in Canada, which will include cloud-based servers. Consent documents need to stress that it is not possible to withdraw data that have already been distributed and used. In particular, patients need to know that researchers will be able to access all updates to their records until they withdraw consent to do so.3,5,9,10,12
Future contact and assent
The option that participants, including mature minors, may be recontacted should be included in consent documents, as well as the option for obtaining consent of children, where applicable. In rare instances, a research project may require future contact and assent, depending on the nature of the project.4,7,9,13,16
Additional considerations
The core set of elements is meant to enable implementation of common language, while maintaining flexibility necessary for components that are not considered core. Research ethics boards may require research teams to address issues that are not part of the core elements per se but have been raised as critical components in some contexts (Table 2).3,5,7,14–21 Any limits to open data sharing should be described in consent documents, which may include mechanisms to allow data sharing, with agreed-upon governance for Indigenous Peoples that supports data sovereignty.22,23 Processes for the return of material incidental findings may also be outlined for those who have opted in to their disclosure, and in some cases, researchers may be required to list any personal identifiers that will be necessary to collect for data-linking purposes.12,14,15 If linking to administrative data is required, consent documents could direct researchers to a guideline developed by the Health Data Research Network (HDRN) Canada.20 Other topics, such as the need to access biospecimens or a clear description of expectations for direct benefit, may also need to be included.
Additional considerations* for human genome research consent documents
These additional considerations have not been added to the core elements because they will depend on the overall aim or governance chosen for the specific research project. Their inclusion in different ways will not harm the interoperability of projects in general or within the context of the CHGL.
Methods
This guideline project was conceived of and managed by the Ethics Office at the Science Policy Branch of the Canadian Institutes of Health Research (CIHR) and the CIHR Institute of Genetics (CIHR-IG), building on national and international consultations held from 2019 to 2021. The timeline and processes used to develop the guidance are outlined in Table 3.
Timeline for the development of the pan-Canadian genome core consent elements
In November 2019, the Canada–UK Clinical Genomic Data Sharing for Research Workshop was held; the primary outcome was that a core set of pan-Canadian genomic consent elements was required to ensure interoperability between genomic data sets, moving forward. As a result of this workshop and further consultations, a working group — chaired by the scientific director of CIHR-IG (C.M.) — was created, which further defined the scope of the project.
Composition of participating groups
This work was made possible through the establishment and participation of 3 groups: a working group, a writing group and an advisory group. The working group comprised 10 key participants, identified through the consultations held in 2019, including clinicians, ethicists, legal experts, patients, chairs of research ethics boards and biomedical researchers. This group developed the project and provided guidance and feedback to the writing group. A subcommittee of the working group, members of the writing group (C.M., E.R., H.L., J.E., J.F., M.Z.), volunteered to draft the current document.
In order to include a comprehensive collection of perspectives, the broader advisory group (40 members) was composed of participants from the various consultations, and of additional national and international stakeholders. Stakeholders included medical and clinical geneticists; genetic counsellors; patients; patient groups; experts in ethics, policy and legal issues; and research funders.
Guideline processes
Preliminary consultations
At the 2-day Canada–UK Clinical Genomic Data Sharing for Research Workshop in 2019, Genomics England presented its 100 000 Genomes Project and distributed its genomic consent form to all attendees.9 In 2020, staff of the CIHR-IG met with Australian Genomics and received copies of its genomic consent. These 2 consent forms were discussed in depth at the first working group meeting. Members of the working group also shared genomic consents developed for various cross-Canada projects (the Care4Rare Consortium, the HostSeq Database and the childhood cancer Precision Oncology for Young People [PROFYLE] project).24–26 These forms served as a common starting point to help the working group identify the initial core elements necessary for a human genomic consent for Canada.
Literature search
From June 2019 to December 2021, the writing group conducted a series of literature searches (including for grey literature), to identify policy, consent and governance documents for genomics research, along with other related literature. In addition to the consent forms mentioned above, key literature, policy and other related documents were made available to the working group,7,9–11,13,21,27–29 as were consent and governance documents identified through the searches.6,9,24–26,30
The work completed under the umbrella of the Global Alliance for Genomics and Health (GA4GH) Regulatory and Ethics Work Stream was also used to inform the development of this guideline,10,30 which describes foundational principles, codes and conventions in addition to policy and regulatory compliance requirements.
Development of recommendations
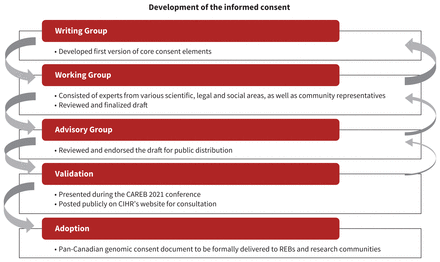
Based on these consultations, existing consent forms and results of the literature searches, the writing group developed an initial draft core set of consent elements by consensus (process shown in Figure 1). After subsequent rounds of consultations and iterations, the writing group and working group came to consensus on the draft elements.
Process used to derive the genomic core consent elements. Note: CAREB = Canadian Association of Research Ethics Boards, CIHR = Canadian Institutes of Health Research, REB = research ethics board.
The broad advisory group reviewed the draft elements, and the working group integrated its input into the core consent elements, after reaching consensus on the suggested edits.
External review
In addition to the work and contribution of the 3 guideline groups, the project was supported by external consultations. The first consultation (E.R., J.R.) was held in February 2021, during the “Engagement Session #5 on Clinical Trial Oversight and Implementation in the Context of COVID-19,” which was hosted virtually by Health Canada in collaboration with CIHR, the Canadian Association of Research Ethics Boards (CAREB), the Secretariat of the Panel on Research Ethics and invited stakeholders. The meeting included direct questions to the participants and allowed the working group to validate the approach it was using.
The second consultation was in the context of the 2021 CAREB annual meeting in May 2021, where the near-final draft was presented (C.M., E.R., J.F.) to those with an interest in Canadian research ethics boards; content of the discussion was summarized and reported back to the writing group, which edited the draft based on those suggestions, after discussion and consensus was reached with the working group.
Finalization of recommendations
A virtual workshop was held on June 23, 2021, comprising all 3 groups (working, writing and advisory), during which final edits were made and a consensus reached on the final wording and scope of the core consent elements. The core consent elements included in this guideline are presented in the final form that was approved at the end of this meeting.
Management of competing interests
In developing this guidance, the writing group adhered to the principles of managing competing interests from the Guidelines International Network.31 All participants were asked to verbally declare any potential competing interests at the start of formal meetings, with the writing group assessing and monitoring the presence of competing interests throughout the guideline development process. None of the members of the writing group, working group or advisory group had direct financial or indirect benefits from the publication of this guideline other than visibility provided as participants.
Implementation
The implementation and deployment of this guideline in the context of the CHGL will follow the development of the library, which will be launched in 2023. Funded by the CIHR and Genome Canada, the CHGL project is being developed in collaboration with the CGEn HostSeq project,25 and benefiting from the establishment of the Digital Research Alliance of Canada.32 The implementation of the core consent elements will be ensured by the establishment of the CHGL governance and processes. Research funders will engage with their communities to promote the uptake of this guideline for the CHGL and for human genomics research in general as applicable. It is also expected that the research ethics boards will encourage the use of this guideline as it has the potential to streamline genomic consent across the country, facilitate multisite projects and simplify the approval process for all those involved. Genetic counselling is beneficial before and after genomic sequencing.33
The writing group will reassess these core elements every 3 years, in consultation with members of the broader advisory group and other stakeholders, to ensure that the language used is in keeping with national and international best practices.
Other guidelines
Harmonized consent forms have been used by genomics and other groups in Canada — including by the Care4Rare Consortium, 24 the PROFYLE project27 and HDRN Canada21 — and internationally. 10,13,30 Others were more recently developed in response to the COVID-19 pandemic, including one by the Canadian COVID-19 Genomics Network (CanCOGeN HostSeq), which permits and streamlines broad sharing of data, showing the importance and impact of having such harmonization.25 Collectively, these consents provided a starting point for the development of these broader pan-Canadian genomic core consent elements.
As noted earlier, we used national consents for human genome sequencing in other jurisdictions, such as Australia and England, in the development process for our core consent elements.9,13,34 However, we gave careful consideration to ensuring that any elements from these consents that we included met the minimum requirements of Canadian research ethics boards, as outlined in the TCPS 2.6
Additionally, recent projects spearheaded by, among others, the Global Alliance for Genomics and Health,35 CanCOGeN25 and HDRN Canada20 have shown the importance of genomic and administrative data consents in allowing national and international data sharing according to predetermined, agreed-upon criteria, while ensuring the security of the data and preserving the privacy of individuals.10 These consents meet the local policy and regulatory needs of data providers and should foster more streamlined data-sharing processes.
Gaps in knowledge
A plethora of academic literature on genomic consents and the need for data sharing exists. Consents in use currently at a national level include those used by Genome England and Australian Genomics, as well as recommendations on national consents from GA4GH.9,10,13,30 However, these are relatively new documents whose intent, ethics, usefulness and uptake by researchers, clinicians, the public and patients will require ongoing study.
Our proposed Canadian guidance is broadly applicable, but additional measures regarding control and access to data will be warranted for some populations (e.g., Indigenous Peoples).17,18,36 More work is required in this area.
Limitations
Although we conducted extensive engagement to create this guideline, additional consultation and subsequent modifications will be required over time to ensure that it remains relevant and compliant with the changing Canadian regulatory and policy landscape. Substantive, continual engagement will also be required with research participants to determine whether the current set of consent elements adequately informs them of risks, benefits and other key information in ways that are meaningful to them.
Sometimes, to ensure actual and future interoperability, common tools that work for most, but not all, are developed.3,10,21,27 It is important to ensure that, in the desire to create consistency across consent elements, communities requiring alternative solutions are not excluded.12,13,22,23 For genomic data to have the widest possible impact, all communities and all people should be able to participate in and benefit from genomic science, should they so choose.37,38 In particular, extensive engagement with Indigenous people and Indigenous-led projects must take place to ensure that the CHGL adequately supports Indigenous Sovereignty and that it benefits all peoples and groups equitably.18,22,23
As currently written and without these future efforts, this guidance could serve to reinforce the current genomic gap, where certain groups are underrepresented or excluded from participation and therefore cannot benefit from genomic and genetic research; this is not the intention of these recommendations.18,22,23,36 At the time of publication of this guidance, discussions are under way to determine optimal approaches that encourage access to research for all.
Conclusion
The adoption of a set of pan-Canadian minimal genomic consent elements that is clear and easy to understand will foster maximum impact of genomic sequencing done in Canada. We believe that the guidance produced here could have wide appeal to the oversight of other domains of research where there are shared intent and design. Using this guideline should also streamline approval of projects by research ethics boards and data providers by ensuring that the core elements of genomic consents are included in reviewed projects. By increasing the capacity to share genomic data across the country, this guideline will allow the development of a structure enabling the federation of genomic data generated in Canada.
Acknowledgements
The authors thank the members of the working and advisory groups for their help in the preparation of this guidance, as well as all those who participated in the external consultations.
Footnotes
Competing interests: Christopher McMaster reports receiving a grant from the Canadian Institutes of Health Research Institute of Genetics (CIHR-IG) in support of the present manuscript, and operating grants from CIHR and Natural Sciences and Engineering Research Council of Canada, outside the submitted work; these grants were also used for travel. Dr. McMaster is the Scientific Director for the CIHR-IG (paid role). Ma’n Zawati reports receiving funding from a Junior 1 Fonds de recherche du Québec Career Award. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and the acquisition, analysis and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Funding for the workshops and meetings that aided in the development of this guideline was provided by the Canadian Institutes of Health Research Institute of Genetics.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/