Abstract
Background: Globally, pharmaceutical companies offer patient support programs in tandem with their products, which aim to enhance medication adherence and patient experience through education, training, support and financial assistance. We sought to identify the proportion and characteristics of such patient support programs in Canada and to describe the nature of supports provided.
Methods: We conducted a crosssectional study to identify and characterize all marketed prescription drugs available in Canada as of Aug. 23, 2022, using the Health Canada Drug Product and CompuScript databases. To describe the nature of supports provided, we conducted a content analysis of publicly available patient support program websites and Web-based documents. Using logistic regression, we identified characteristics of drugs associated with having a patient support program including brand-name or branded generic (generic medications with a proprietary name), orphan (medications for rare diseases) or biologic drug status; estimated total cost of prescriptions dispensed at retail pharmacies; and price per unit.
Results: Of the 2556 prescription drugs marketed by 89 companies in the study period, 256 (10.0%) had a patient support program in Canada. Many of the 89 drug manufacturers (n = 55, 61.8%) offered at least 1 patient support program, frequently relying on third-party administrators for delivery. Brandname and branded generic medications, biologic agents and drugs with orphan status were more likely to have a patient support program than generic drugs. Compared with drugs priced $1.01–$10.00 per unit, drugs priced $10.01–$100.00 per unit were nearly 8 times more likely to have a patient support program (adjusted odds ratio 7.54, 95% confidence interval 4.07– 14.64). Most sampled patient support programs included reimbursement navigation (n = 231, 90.2%) and clinical case management (n = 223, 87.1%).
Interpretation: About 1 in 10 drugs marketed in Canada has a manufacturersponsored patient support program, but these are concentrated around brand-name, branded generic, biologic and high-cost drugs, often for rare diseases. To understand the impact of patient support programs on health outcomes and sustainable access to cost-effective medicines, greater transparency and independent evaluation of patient support programs is necessary.
“If you’re playing in the specialty medicines field,” argued a pharmaceutical industry consulting firm, “a patient support program is the price of admission.”1 Pharmaceutical company–sponsored patient support programs, designed to lessen financial and clinical barriers for patients and prescribers to starting and sustaining treatment, exist in high- and middle-income countries globally.2–7 Once prescribed the treatment, patients are referred to the program by their health care provider or they may self-enroll. They are then contacted by a program coordinator, typically a registered nurse who may help the patient navigate insurance coverage options, coordinate home drug delivery, teach self-injection techniques, answer questions on an on-call basis and conduct follow-up to support patient treatment adherence.8–10 Neither patients nor insurers pay for these services; thus, the cost of the medicine likely includes these supports.
In an era where policy-makers are grappling with escalating drug prices and budgetary impacts globally,11 the pharmaceutical industry promotes patient support programs as adding complementary value to a drug through supporting medication adherence and enhancing clinical outcomes, patient experience or quality of life.3 Industry stakeholders have also identified patient support programs as a valuable opportunity to collect patient-level data as a means to evaluate clinical, quality-of-life and economic outcomes, and, thereby, define a drug’s value to payers more clearly.12
Because patient support programs are proprietary, understanding of these programs and their outcomes relies on studies funded and conducted by the drug’s manufacturer using proprietary patient data collected through the patient support program. These studies are typically focused on the evaluation of patient-reported outcomes (e.g., adherence, persistence) and economic impacts (e.g., health resource use), and usually report positive outcomes,3,13,14 as seen in a longitudinal study of patients in Canada prescribed adalimumab and enrolled in the manufacturer’s patient support program, AbbVie Care.15,16
Other details about the types of supports offered by patient support programs have emerged from litigation. In 2020, AbbVie settled a California suit in which the state alleged that the patient care and insurance authorization assistance provided by the nurses of the patient support program constituted a kickback because it provided “free and valuable professional goods and services to physicians,” contingent upon prescription of the drug.17,18
Overall, industry-sponsored patient support programs and the extent or nature of the services provided are not well understood,17 making it difficult to assess their value to patients or their impact within health systems. Canada offers a useful case study to conduct a national survey of industry-sponsored patient support programs. Patient support programs began to appear in Canada when biologics first came on the market in the early 2000s.9 Funded by drug manufacturers, patient support programs are typically administered by third-party service providers.19
Canada has among the highest drug prices and per-capita spending on biologics among Organization for Economic Co-operation and Development (OECD) countries and, for many products, lower uptake of biosimilar medicines, which are cost-effective alternatives to biologics.20,21 The extent to which patient support programs are offered for biologics and nonbiologics, or for biosimilar or generic drugs is unknown. We sought to identify the proportion and characteristics of marketed prescription drugs available in Canada that had accompanying manufacturersponsored patient support programs and the prevalence and nature of supports provided.
Methods
Study design
We conducted a cross-sectional study to quantify the proportion of prescription drugs with a patient support program on the Canadian market as of Aug. 23, 2022, and describe their characteristics. We defined a patient support program as services (including but not limited to financial assistance) offered to patients prescribed a specific drug that were started and funded by the manufacturer.3,4 We then conducted a structured content analysis of Web-based sources to understand the types and range of supports provided to patients through these programs.
We chose to rely exclusively on publicly available data sources to identify and describe manufacturer-sponsored patient support programs as these are sources currently available to patients when making program enrolment decisions and to policy-makers seeking to understand the extent and impact of this model of care.
We report the study according to the Strengthening the Reporting of Observational studies in Epidemiology checklist.22
Setting
Specialty medicines are characterized as highly complex and high cost, and have complicated handling, storage, administration and monitoring regimens that often require the involvement of nurses and pharmacists.23 Many are manufactured in laboratory-grown cells and are known as biologics.24 Typically priced at more than $10 000 for a 1-year course of treatment, specialty medicines (biologics in particular) account for an increasing share of public and private drug spending.20
Sampling frame
Because the European Medicines Association defines a patient support program as services for a specific drug offered by the company holding the marketing authorization,3 we first sought to identify all drug companies with currently marketed prescription products in Canada. Between June 27, 2022, and Aug. 23, 2022, 2 authors (A.Q. and D.H.) independently extracted the names of all member companies listed on the websites of the 3 main trade associations for the Canadian pharmaceutical industry, namely Innovative Medicines Canada, representing the research-based pharmaceutical industry (typically including manufacturers of brand-name medications);25 BIOTECanada, representing the biotechnology industry;26 and the Canadian Generic Pharmaceutical Association, representing generic drug manufacturers.27 Because trade association membership is voluntary, we supplemented this list with nonmember drug manufacturers identified in previous research by an author (J.L.)28
Using the Health Canada Drug Product Database,29 2 authors (A.Q. and D.H.) independently screened the list of companies and included those with marketed prescription products. We excluded companies that were not drug manufacturers (e.g., law firms) and those without marketed prescription drugs (e.g., products under development) at the time of the study. Screeners resolved discrepancies through discussion or adjudication by a third author (Q.G.).
Sample and variables
Using the Health Canada Drug Product Database,29 1 investigator (A.Q. or D.H.) searched each identified drug manufacturer and extracted the product and active ingredient names for all marketed prescription drugs. We counted a single drug as all dosages, formulations or routes of administration with the same active ingredients and manufacturer since industry patient support programs are brand-specific and do not typically differentiate among these factors.
We selected and extracted variables that reflected known characteristics of drugs and that may be associated with having a patient support program,3,19 including brand status, biologic status, orphan drug status (i.e., whether the drugs are for rare diseases), route of administration, therapeutic indication, estimated total cost of and number of prescriptions dispensed at retail pharmacies (a measure of market share) and price per unit.
On the basis of the type of Health Canada regulatory review,30 clinical expertise and knowledge about the manufacturer, 2 authors (Q.G. and M.T.) independently identified the brand status of each drug as brand-name (i.e., innovator products first to market), branded generic (i.e., subsequent entry products that contain identical medicinal ingredients or are highly similar to an existing product on the market, but given a proprietary name by the manufacturer) or generic (i.e., subsequent entry products that contain identical medicinal ingredients to an existing product on the market, but not given a proprietary name by the generic manufacturer). We classified biosimilars, which are biologic drugs that are highly similar to an existing product on the market,31 as branded generic drugs. We resolved discrepancies through discussion or adjudication by a third author (J.L.), as required.
Using the Health Canada Drug Product Database and the drug’s product monograph,32 1 investigator (A.Q. or D.H.) extracted verbatim routes of administration, the Level 1 Anatomic Therapeutic Chemical (ATC) code and whether the drug is a biologic and thus listed as Schedule D of the Food and Drug Act,33 meaning the drug comes from living organisms or from their cells. The investigator also identified whether the drug had orphan drug status, meaning the drug was indicated for a life-threatening, seriously debilitating or serious and chronic condition affecting a fairly small number of patients and, depending on the jurisdiction, may be subject to an adapted regulatory pathway, or eligible for tax incentives or additional market exclusivity.34,35 Although Health Canada has reported approvals of orphan drugs since 2017, we used the searchable United States Food and Drug Administration Orphan Drug Designation database, which includes approvals since 1983, to identify these drugs.36
Using national dispensing data from IQVIA’s Canadian CompuScript database, 1 investigator (A.Q., D.H. or S.C.) extracted each drug’s estimated total cost and number of prescriptions dispensed at retail pharmacies in Canada for the year 2021. The estimated total cost of prescriptions reflects the sum of all estimated costs of the prescriptions dispensed by community pharmacists, including pharmacy mark-up and dispensing fees. The total number of units sold represents the number of standardized units based on the most common purchasing formats (e.g., tablets, capsules, mL) for total prescriptions dispensed. We calculated the price per unit for each drug by dividing the estimated total cost of prescriptions for all formulations of the drug by the estimated total number of prescription units for all formulations of the drug dispensed in 2021.
The CompuScript database includes only drugs dispensed through retail pharmacies (i.e., does not include drugs administered in hospital), and manufacturers can opt out of data collection. However, the CompuScript database does not provide specific reasons why data are missing. If we could not identify the estimated total cost and number of prescriptions for a sampled drug in the database, 2 investigators with clinical knowledge (Q.G. and M.T.) independently judged likely reasons (e.g., low prescription counts, recent market entry) that price per unit data were missing from the CompuScript data to provide readers additional context.
Identifying patient support programs and their characteristics
We identified whether a drug in our sample had an associated manufacturer-sponsored patient support program for patients in Canada. Based on recent systematic and comparative reviews of patient support programs in North America and Europe,3,4 and an exploratory, empirical study in Australia,5 we defined a patient support program as any combination of services or resources related to medication access, administration, adherence, education, storage or disposal for patients prescribed a specific product and started and sponsored or operated by the company holding the product’s marketing authorization.
We distinguished patient support programs from patient assistance programs, choosing to exclude patient assistance programs because they exclusively provide financial assistance (e.g., coupons, co-pay coverage) and no other categories of supports, and are considered a distinct pharmaceutical company activity.3,4,37 We also excluded expanded access or compassionate access programs, risk management programs outlined in the product monograph (required by the regulator rather than started by the manufacturer) and programs delivered solely for a clinical study.
Building on effective methods for sampling industry Internet documents,38,39 2 authors (A.Q. and D.H.) independently performed structured searches on Google (“[company name] AND patient support program AND Canada” and “[drug brand name] AND patient support program AND Canada”) to identify industrysponsored patient support programs in Canada, resolving discrepancies through discussion, with a third author (Q.G.) adjudicating any outstanding discrepancies.
Using Zotero, a reference management software, 2 authors (A.Q. and D.H.) independently downloaded and catalogued public-facing web pages and documents (e.g., web pages for the program, patient portals and apps, educational materials, press releases, enrolment forms) that explicitly mentioned the patient support program, the sponsoring company and the specific drug, and were intended specifically for a Canadian audience. The authors met to reconcile any discrepancies, with another (Q.G.) adjudicating as necessary. We excluded web pages directed exclusively at health professionals.
Using REDCap,40 we created a data extraction form based on the existing empirical research describing patient support programs (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230841/tab-related-content).3–5 We extracted characteristics of the sampled patient support programs, including target population (adult, pediatric or both), evidence of third-party administration, the nature of supports offered (including financial assistance, reimbursement navigation, injection training, infusion coordination, education, clinical case management, pharmacy services and material resources) and other relevant details (e.g., modalities, availability and access, clinician involvement). Because the definition of a patient support program continues to evolve within the literature and no expert recommendations or jurisdictional regulations are available to guide the development, components or administration of patient support programs,3 we included the option to select and specify other types of supports to ensure comprehensiveness. Coders were prompted to extract, verbatim, illustrative evidence for the presence of a particular type of support.
Two authors (A.Q. and D.H.) independently piloted the data extraction form on a random sample of 10% of the patient support programs. Through discussion (Q.G., A.Q., D.H.), we resolved all discrepancies and refined the data extraction form to ensure consistency. The remainder of the sample was coded by a single author. Because we did not validate these data with drug manufacturers directly, we coded variables dichotomously as either having evidence of the existence of particular supports or no information.
Data analysis
We conducted a descriptive analysis on the full sample of marketed prescription drugs, generating crude descriptive statistics using frequencies and percentages for categorical characteristics. Based on the distribution of the data, 2 authors (Q.G. and M.T.) categorized variables, merging categories with very small sample sizes, including merging Level 1 ATC codes into 7 categories, grouping them by broad physiologic system or clinical area into other (sensory organs, various, dermatologicals, and musculoskeletal system); antiparasitics and anti-infectives; genitourinary and hormones; nervous system; cardiovascular, blood and respiratory; alimentary tract and metabolism; and antineoplastic and immunomodulating agents. We also grouped medications by route of administration into 3 categories (oral, injection and other), coding drugs with multiple formulations according to the most common route of administration. If more than 1 route was commonly used, we coded for the most complex route, defining this as the route of administration requiring the greatest clinical support (e.g., intravenous, subcutaneous).
Based on the data distributions, we also categorized estimated total cost (i.e., a measure of market share) of prescriptions dispensed at a retail pharmacy and price per unit into 4 categories (e.g., price per unit < $1, $1.01–$10.00, $10.01–$100.00, ≥ $100.01). These costs were calculated for calendar year 2021.
We conducted logistic regression analyses to assess the relationship between having a patient support program and a drug’s characteristics. Because the CompuScript database includes only drugs dispensed through retail pharmacies and manufacturers can opt out of data collection, some drugs had missing data for estimated total cost of prescriptions and price per unit; we excluded these drugs from the regression analyses. We conducted univariable logistic regression analyses to assess the relationship between having a patient support program and a drug’s characteristics, including brand, biologic or orphan drug status, ATC classification (Level 1), route of administration, estimated total cost of prescriptions dispensed at retail pharmacies and price per unit. We also conducted a multivariable logistic regression analysis to assess how the presence of these drug characteristics reflected the existence of a patient support program. We assessed multicollinearity among predictor variables in the multivariable regression using the variance inflation factor, whereby values that exceed 5 or 10 indicate a problematic amount of collinearity.41 Because generic drugs are more numerous and more likely to be lower cost, we conducted 2 sensitivity analyses replicating the univariable and multivariable logistic regression models for only brand-name drugs, and then only brand-name and branded generic drugs. In all logistic regression models, we reported the odds ratio (OR) with profile or likelihood-based 95% confidence intervals (CIs).
We conducted a directed content analysis to describe the prevalence and nature of supports offered through identified patient support programs.42 Based on the literature describing patient support programs3–5 and the extracted data, we (Q.G., A.Q., D.H., J.L., M.T.) deductively derived 6 broad categories of support (i.e., financial, clinical, educational, pharmacy, material or not specified). The team, through discussion and review of extracted data, inductively derived subcategories or types of support within each broad category. Two investigators (D.H., A.Q. or Q.G.) then independently reviewed the extracted data and source materials for each program to dichotomously code for evidence of each category of support or whether the program had no specified supports. Investigators resolved discrepancies through discussion or adjudication by a third author (D.H., A.Q. or Q.G.). We calculated the prevalence for each category and subcategory of supports, and selected verbatim examples from the coded programs to qualitatively illustrate the nature and range of supports.
Ethics approval
This study did not include human participants or their data and thus was exempt from ethics review as per the University of Toronto Health Sciences Research Ethics Board.
Results
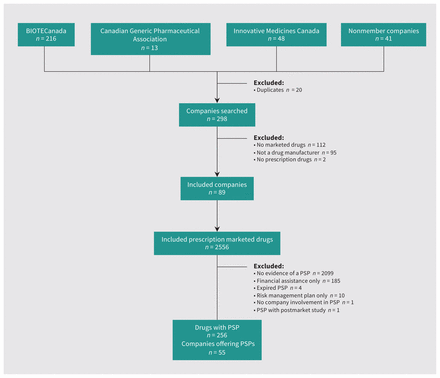
We identified 2556 prescription drugs marketed by 89 companies, including all prescription drugs administered in hospital and outpatient locations. We identified evidence of an accompanying patient support program for 256 (10.0%) marketed prescription drugs; 55 (61.7%) of the 89 companies offered a patient support program (Figure 1). Nearly all of the 263 data sources describing patient support programs were created and disseminated by the sponsoring manufacturer (n = 249, 94.7%), such as dedicated websites, press releases, enrolment forms and brochures. Patient associations or hospitals authored and published the materials identifying and describing the other 14 (5.3%) patient support programs.
Flow diagram for identifying patient support programs (PSPs).
Characteristics of all 2556 marketed prescription drugs, with and without patient support programs, are outlined in Table 1. Most drugs were generic (n = 1535, 60.1%) and administered orally (n = 1647, 64.4%). A relatively small proportion of marketed prescription drugs were biologics (n = 251, 9.8%), had orphan drug status (n = 275, 10.7%) or were a biologic with orphan drug status (n = 102, 3.9%).
Characteristics of drugs with and without patient support programs (PSPs)
More than half of the 256 drugs with a patient support program were biologics (n = 138, 53.9%) or had orphan drug status (n = 118, 46.1%); one-quarter had both designations (n = 67, 26.2%). Most drugs with associated patient support programs had original market dates after 2012 (n = 183, 71.5%), with 104 (40.6%) marketed after 2018. Most drugs with a patient support program were indicated for adult populations only (n = 168, 65.6%).
Data on estimated total cost of prescriptions were available for 2214 drugs dispensed through retail pharmacies, including 210 (82.0%) of 256 drugs with a patient support program and 2004 (87.1%) of 2300 drugs without a program. Among drugs with missing data were those dispensed only in hospital (n = 126), blood products (n = 52), drugs with very low prescription counts (n = 45) and those with recent market entry, after 2021 (n = 9).
Of the 2214 drugs dispensed through retail pharmacies, most (n = 1632, 73.7%) cost $10.00 per unit or less. Drugs with a patient support program had a median price per unit of $208.4 (interquartile range [IQR] $38.1–$716.3) versus $1.47 (IQR $0.58–$6.51) for drugs without programs. Figure 2 shows the distribution of patient support programs for high-cost drugs (≥ $100.01 per unit, n = 222).
Number and proportion of patient support programs (PSPs) by cost for high-cost drugs (n = 222).
The 256 drugs with patient support programs represented 234 unique combinations of active ingredients. For 22 of the active ingredient combinations, 72 patient support programs were offered by different manufacturers for the same therapeutic indications, including adalimumab (n = 7), fingolimod hydrochloride (n = 6), rituximab (n = 5), dimethyl fumarate (n = 5), infliximab (n = 4) and teriflunomide (n = 4) (Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230841/tab-related-content).
Table 2 presents the results of univariable and multivariable logistic regression models, predicting the likelihood of drugs dispensed through retail pharmacies having a patient support program. The univariable and multivariable regression models included 2210 drugs dispensed through retail pharmacies with complete data for all drug characteristics, excluding 4 outlier drugs with prices per unit greater than $14 000. Like the univariable analysis, the multivariable logistic regression showed that brand-name, biologic and orphan drugs, and those with higher prices per unit, were more likely to have associated patient support programs. In our multivariable regression model, all variance inflation factors were less than 1.5, suggesting no evidence of collinearity.
Univariable and multivariable logistic regression for the association between presence of a patient support program and drug characteristics (n = 2210)
Compared with drugs priced $1.01–$10.00 per unit, drugs priced $10.01–$100.00 were 8 times more likely (adjusted OR 7.54, 95% CI 4.07–14.64) to have a patient support program; drugs costing $100.01 or greater per unit were 11 times more likely (adjusted OR 10.58, 95% CI 5.10–22.72) to have a patient support program. Sensitivity analyses excluding generic drugs were consistent with our main results (Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230841/tab-related-content).
Characteristics of drug manufacturers and third-party administrators
The 256 patient support programs were funded or delivered by 55 (61.7%) of the 89 companies. Of those offering patient support programs, most were members of the brand-name drug manufacturers or biotechnology trade associations or both (n = 38, 69.1%). For half (n = 128, 50.0%) of the 256 patient support programs, we found evidence that manufacturers contracted a third-party to administer or deliver the patient support program (Table 3). Two companies, Innomar Strategies and McKesson Canada, accounted for more than 40% of third-party delivery (n = 54, 42.2%).
Third-party patient support program administrators
Characteristics of patient support programs
Table 4 outlines the type and prevalence of supports offered by patient support programs. Most sampled patient support programs included financial assistance or reimbursement navigation (n = 231, 90.2%), or clinical case management provided by a nurse (n = 223, 87.1%).
Types and frequency of supports offered within patient support programs (PSPs)
Interpretation
We identified and studied 256 industry patient support programs, which accompanied about 10% of marketed prescription drugs. Most sampled pharmaceutical companies (61.7%) offered patient support programs for their marketed drugs, including members of the research-based, biotechnology and generics industries. Patient support programs were concentrated among brand-name, branded generic, biologic and high-cost drugs, often for rare diseases.
Our finding that 10% of marketed prescription drugs had an accompanying manufacturer-sponsored patient support program is likely an underestimate, given our reliance on materials available to the public and patients; for example, we may have missed patient support programs for drugs that are highly specialized and used in rare instances, and thus may not be publicly advertised. A 2023 report published by 20Sense, a specialty medicines consulting company in Canada, estimated that 400 patient support programs were available in Canada, citing unpublished research;19 however, they did not explicitly define what constituted a patient support program.
Recent reviews — including a systematic review of 20 studies of 8 industry patient support programs in Europe3 and a scoping review of 70 studies of 56 patient support programs offered by industry, government and health care organizations globally52 — have synthesized findings on the types of supports offered within programs. However, these reviews examined only patient support programs described within the peer-reviewed literature. Although previous reviews documented heterogeneity among patient support programs,3,52,53 our study found that patient support programs typically included financial supports (including reimbursement navigation) and nursing care in the form of case management, health teaching and counselling — although the degree and intensity of service provision is an important question for future research. Compared with a previous review,3 our study found a higher prevalence of clinical supports, including nursing care and pharmacy coordination, suggesting the value of this type of care to patients, prescribers and payers.
These findings have several policy implications. The provision of these supports may have inefficiencies. Our study documented the duplication of services across companies marketing drugs with the same active ingredients. In the context of global shortages in health human resources, the impact of patient support programs on health human resources should be considered.
The prevalence and nature of patient support programs lack public transparency. As proprietary offerings generating proprietary data, the impact of these programs is currently not clear to decisionmakers. Thus, decision-makers may find it challenging to independently evaluate value for money and health system impacts, including access to medicines and medication-related care.
Finally, although manufacturers may be filling important gaps within publicly funded health systems,9,19 whether manufacturer-sponsored patient support programs are the optimal model to address health needs related to medicines is an open question. The delivery of health care should be organized around a health need, not a particular therapeutic product. For example, participants in an Australian study endorsed the value of holistic nursing care for chronic disease within the health system rather than referral to multiple industry patient support programs.5 In 2007, the French government commissioned an independent investigation into patient support programs, which suggested that direct contact between the pharmaceutical industry and the public be prohibited because of role confusion and misaligned incentives.54
Despite these policy concerns, few documented policy responses have addressed the regulation of industry patient support programs, a challenge exacerbated by the lack of transparency around their prevalence or activities. In 2009, the French government passed a law, in response to the independent investigation, that formalized industry patient support programs, requiring approval by the health regulator and prohibiting the involvement of company representatives; programs could instead be implemented by industry-sponsored clinicians.55 In the United States, the government has taken legal action against pharmaceutical companies at the state and federal level under the Anti-Kickback Statute, alleging that the services provided under patient support (e.g., nursing services) and patient assistance programs (e.g., cost-sharing mechanisms) constitute an inducement to providers or patients to use a particular drug kickback; however, legal cases have been settled out of court or remain pending, and no additional regulation has been imposed on these activities to date.10,56
No literature exists on the attitudes of patients, health care providers, payers or policy-makers toward these programs or their experiences navigating care systems that involve patient support programs, suggesting an important avenue for future work. Studies of the prevalence and characteristics of patient support programs in other jurisdictions would provide useful comparative information to understand what might be unique to the Canadian context or the extent to which the global pharmaceutical industry employs patient support programs.
Limitations
The cross-sectional design and reliance on publicly available sources mean we may have missed drug manufacturers or their marketed drugs. However, we conducted all sampling and searches for patient support program data in duplicate, triangulating several search strategies and Web-based sources; thus, it is likely that any missed medicines are those without patient support programs during the study period.
Because the definition of a patient support program is evolving,3 our identification of patient support programs is 1 possible interpretation. The study relied on publicly available documents; since we did not verify this information with companies, we may have missed some supports offered by a patient support program or incorrectly classified a program as a patient assistance program if we found evidence of only financial supports. However, although we relied on publicly available information, most included data sources were manufacturer-produced content, primarily web pages, brochures and enrolment forms, and coding for the presence and absence of supports was done in duplicate, using the source materials for verification.
The European Medicines Agency classifies some therapeutics as orphan drugs that do not receive this classification from the US Food and Drug Administration and, therefore, we may have undercounted the number of orphan drugs approved by Health Canada. Finally, we lacked information regarding the discounts that manufacturers extend to payers and buyers, as these agreements are secret. These reductions in cost can be quite substantial. However, our findings encompass the entirety of purchases within the drug system and total spending is still a good approximation of the market size; we anticipate that drugs that were categorized as high cost or having large market sizes would still fall into the same categories if all rebates were considered.
Conclusion
Industry-sponsored patient support programs routinely offer financial, clinical and educational supports to patients, and are primarily available for high-cost drugs. To understand the impact of patient support programs on patient and public health outcomes, and sustainable access to cost-effective medicines, greater transparency and independent evaluation of patient support programs is necessary.
Footnotes
Competing interests: Joel Lexchin received payments for writing briefs on the role of promotion in generating prescriptions for 2 legal firms. He is a member of the Foundation Board of Health Action International and the Board of Canadian Doctors for Medicare. He receives royalties from University of Toronto Press and James Lorimer & Co. Marc-André Gagnon reports research funding from the Social Sciences and Humanities Research Council, Canadian Institutes of Health Research (CIHR) and the Faculty of Public Affairs at Carleton University. Mina Tadrous reports research funding from the Ontario Ministry of Health and CIHR, as well as consulting fees from the Canadian Agency for Drugs and Technologies in Health, Health Canada and Green Shield Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Quinn Grundy conceived the idea. Quinn Grundy, Dana Hart, Joel Lexchin, Marc-André Gagnon and Mina Tadrous designed the study. Quinn Grundy, Ashton Quanbury, Dana Hart and Shanzeh Chaudhry contributed to data acquisition. Quinn Grundy, Ashton Quanbury, Dana Hart and Farideh Tavangar performed the analysis. All of the authors were involved in data interpretation. Quinn Grundy drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the Bertha Rosenstadt Research Fund, Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto and the Social Sciences and Humanities Research Council (grant no. 435230050). The IQVIA data were purchased using funding from the Canadian Institutes of Health Research (no. 202107). The funders had no role in the study design; collection, analysis and interpretation of data; or writing of the report; nor did the funder place any restrictions regarding the submission of the report for publication. The researchers were fully independent from the funders and all authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing: All data underlying the analysis are publicly available with no restrictions at doi.org/10.5683/SP3/LYCQUR, except for variables related to estimated total cost of prescriptions dispensed at a retail pharmacy and price per unit in 2021. Variables related to estimated total cost of prescriptions and price per unit were obtained under licence from IQVIA Canada. The raw data cannot be publicly shared as it was obtained from a third party and as per signed agreement. Requests for data can be sent to IQVIA Solutions Canada and may carry a cost.
Disclaimer: The statements, findings, conclusions, views and opinions contained and expressed in this paper are based in part on data obtained under license from the following IQVIA Solutions Canada information service: CompuScript database (all rights reserved). The statements, findings, conclusions, views and opinions contained and expressed herein are not necessarily those of IQVIA Solutions Canada or any of its affiliated or subsidiary entities.
- Accepted September 25, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/