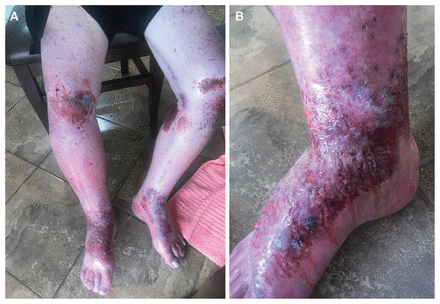
A 61-year-old man with a history of congestive heart failure, atrial fibrillation, chronic kidney disease and hypothyroidism presented to clinic with a painful, bilateral, nonblanching, lower extremity rash 2 weeks after starting empagliflozin (5 mg/d) (Appendix 1, Supplementary Appendix 1 and 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220934/tab-related-content). One month after starting the drug, we increased the dosage to 10 mg/d for ongoing heart failure management; his rash then worsened (Figure 1). Laboratory investigations showed that the patient was positive for antinuclear antibodies (1:160 titre), with no extractable nuclear antigen antibody. Test results for hepatitis B and C serology, antineutrophil cytoplasmic antibodies, cryoglobulins and rheumatoid factor were negative. Eosinophilia was absent and urinalysis was negative for blood with trace protein. The patient was started on 0.05% clobetasol cream, but was subsequently admitted to hospital owing to progression of the rash.
Photographs of a 61-year-old man with bilateral lower limb distribution of drug eruption associated with empagliflozin, predominantly over the knees and feet.
The differential diagnosis for the patient’s rash included vasculitis, cellulitis, contact dermatitis, venous stasis and a cutaneous drug reaction. Our patient had a skin biopsy, which showed perivascular dermatitis with eosinophils and superficial excoriation, consistent with a drug eruption (Appendix 1, Supplementary Appendix 3). Empagliflozin was stopped and the patient completed a 5-day course of 30 mg prednisone with resolution of the rash (Appendix 1, Supplementary Appendix 4). Based on the temporal relationship between starting empagliflozin and the appearance of the rash — which worsened with an increase in dose and resolved when the drug was stopped — and with the exclusion of other medication culprits, we graded our patient’s rash as 8 on the Naranjo algorithm and probable or likely on the World Health Organization Uppsala Monitoring Centre (WHOUMC) causality system, indicating a probable adverse drug reaction to empagliflozin (Appendix 1, Supplementary Appendix 5).
Although sodium–glucose cotransporter-2 (SGLT2) inhibitors, including empagliflozin, are widely used in clinical practice, few cutaneous drug reactions have been reported. Ipragliflozin, which is not available in Canada, has some reports of association with a fixed drug eruption.1,2 Similarly, a postmarketing study using pharmacovigilance databases found a signal for skin toxicity with all SGLT2 inhibitors.3 Clinicians should be aware of this potential adverse event as usage of SGLT2 inhibitors continues to increase.
Clinical images are chosen because they are particularly intriguing, classic or dramatic. Submissions of clear, appropriately labelled high-resolution images must be accompanied by a figure caption. A brief explanation (300 words maximum) of the educational importance of the images with minimal references is required. The patient’s written consent for publication must be obtained before submission.
Footnotes
Competing interests: Kevin Yau reports funding from the Eliot Phillipson Clinician Scientist Training Program (Department of Medicine, University of Toronto), the Banting and Best Diabetes Centre postdoctoral fellowship and the KRESCENT postdoctoral fellowship from the Kidney Foundation of Canada. He has also received speaker fees from AstraZeneca. Stephanie Poon reports funding from Boehringer Ingelheim; consulting fees from Novartis, AstraZeneca and Boehringer Ingelheim; and honoraria from the Canadian Collaborative Research Network, Agence L.I.V. Inc., University of Toronto, University Health Network, Novartis, Boehringer Ingelheim and AstraZeneca. Bourne Auguste has received speaker fees from Baxter and Amgen. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/