Abstract
Background: The cardiothoracic ratio (CTR) is commonly assessed on chest radiography for detection of cardiac chamber enlargement, but the traditional cutpoint of 0.5 has low specificity. We sought to evaluate the diagnostic accuracy of new measurement techniques for the detection of cardiac enlargement on chest radiographs.
Methods: We obtained retrospective cross-sectional data on consecutive patients who underwent both chest radiography and cardiac magnetic resonance imaging (MRI) within a 14-day interval between 2006 and 2016 at a large academic hospital network. We established the presence of cardiac chamber enlargement using cardiac MRI as the reference standard. We evaluated the diagnostic performance of different techniques for measuring heart size and CTR on frontal chest radiographs.
Results: Of 152 patients included, 81 (53%) were men and the mean age was 52 years. Maximum heart diameter had the highest area under the receiver operating characteristic curve for detection of cardiac enlargement (0.827, 95% confidence interval 0.760–0.894). In the subgroup of posteroanterior chest radiography studies (n = 101), a CTR cutpoint of 0.50 had only moderate sensitivity (72%) and specificity (72%). In men, a maximum heart diameter cutpoint of 15 cm had a sensitivity of 86% and a negative likelihood ratio of 0.24, and a cutpoint of 19 cm had a specificity of 100% and a positive likelihood ratio of infinity. In women, a maximum heart diameter cutpoint of 13 cm had a sensitivity of 91% and a negative likelihood ratio of 0.15, and a cutpoint of 17 cm had a specificity of 91% and a positive likelihood ratio of 3.5.
Interpretation: A traditional CTR cutpoint of 0.5 has limited diagnostic value. Simple heart diameter measurements have higher diagnostic performance measures than CTR.
Cardiac chamber enlargement is important to identify, given that it is a predictor of poor outcomes and may reflect potentially treatable underlying disease.1 Cardiac chamber size can be assessed using multiple imaging modalities, including chest radiography, echocardiography, computed tomography and cardiac magnetic resonance imaging (MRI).2–4 Although cardiac MRI is considered the reference standard for the evaluation of cardiac size and function, it is rarely performed as an initial investigation because of its relatively high cost and limited availability.5 On the other hand, chest radiography is frequently performed as the initial imaging investigation for suspected pulmonary and cardiac disease, including in patients presenting with shortness of breath and chest pain.6,7 Therefore, accurate and reproducible measures of cardiac enlargement on chest radiography would help to identify patients with underlying cardiac disease who might benefit from further investigation.
Cardiac enlargement is frequently evaluated on chest radiography using the cardiothoracic ratio (CTR), which was originally described in 1919.8 Although this sign is an accepted and frequently used marker of cardiac enlargement, it has not been validated against cardiac MRI or other contemporary methods of objectively assessing cardiac chamber size. Some studies have shown that the CTR, as assessed using the originally described cutpoint of 0.5, has low specificity and diagnostic accuracy for cardiac enlargement, and therefore may be of limited clinical utility.9–11 The purpose of this study was to evaluate new measurement techniques and cutpoints for the detection of cardiac chamber enlargement on chest radiography in comparison with MRI.
Methods
Study design
We conducted a retrospective, cross-sectional study using a medical imaging database of patients from 3 sites of an academic hospital network (University Health Network, Toronto, Ontario). We included consecutive adult patients (≥ 18 yr) who had undergone chest radiography, contrast-enhanced chest computed tomography and cardiac MRI within a 14-day interval between August 2006 and August 2016. Exclusion criteria included congenital heart disease, incomplete MRI, presence of more than a small pericardial effusion on MRI and inability to measure heart diameter on chest radiography because of large pleural effusions.
Chest radiography and measurements
Chest radiography was performed using conventional radiography equipment and standard patient positioning. Posteroanterior (PA) chest radiography was performed in the upright position at full inspiration with a source-to-target (focal spot-to-film) distance of approximately 180 cm. Portable anteroposterior (AP) chest radiography was typically performed in emergency department or inpatient settings when patients were unable to complete standard PA chest radiography, either upright or supine.
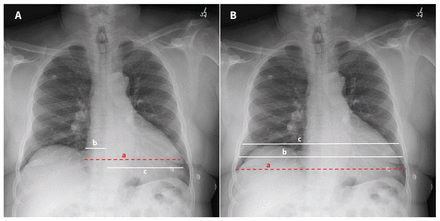
A radiologist with fellowship training in cardiothoracic imaging analyzed radiographs offline, blinded to all identifying information and cardiac MRI results. The radiologist assessed heart size using the maximum transverse diameter, assessed as a single measurement, and as the sum of the maximum distance from the midline to the right and left heart borders (Figure 1A). Thoracic size was measured between the inner margins of the ribs at the dome of the right hemidiaphragm, at the level of the mid heart and at the maximum diameter at any level (Figure 1B). The radiologist calculated 6 separate CTRs by dividing each heart measurement by each thoracic measurement. They evaluated images for rotation, which was scored as present if the distance between the medial ends of the clavicles and the spinous processes of thoracic vertebral bodies differed by greater than 1 cm between the right and left sides.12 The degree of inspiration was also assessed and was scored as limited inspiration if the right tenth posterior rib was not visualized above the dome of the right hemidiaphragm.13
Representative heart (A) and thoracic (B) measurements on an upright posteroanterior chest radiograph in a 61-year-old man with chest pain. (A) Heart size was assessed using the maximum transverse diameter, assessed as a single measurement (red dashed line, a), and the sum of the maximum distance from the midline to the right and left heart borders (white lines, b plus c). (B) Thoracic size was measured between the inner margins of the ribs at 3 levels: at the maximum diameter at any level (red dashed line, a), at the level of the mid heart (white line, b) and at the dome of the right hemidiaphragm (white line, c). Measurement techniques depicted using red dashed lines on A and B are those suggested for assessment of heart and thoracic size, respectively.
To assess interobserver agreement, a second fellowship-trained observer reanalyzed a random subset of 50 studies, blinded to the results of the initial assessment and all identifying data.
Reference standard
Cardiac MRIs were performed at 1.5 T or 3 T (MAGNETOM Avanto or Skyra; Siemens Healthineers). Ventricular and atrial size were assessed using short-axis and 4-chamber, cine balanced steady-state free precession (bSSFP) images, respectively (slice thickness 6–8 mm, 0–2 mm gap, in-plane resolution 1.4–2.0 mm and temporal resolution 30–40 ms).
A radiologist with fellowship training in cardiothoracic imaging performed cardiac MRI analysis, blinded to the results from the chest radiographs and all clinical information. Postprocessing was performed offline using commercially available software (QMASS, Medis Suite v3.0.18.6, Medis Medical Imaging Systems), in accordance with current guidelines.14 Left and right ventricular endocardial borders were contoured for assessment of end-diastolic volumes, which were indexed to body surface area. Maximum left and right atrial cross-sectional areas were assessed at ventricular end-systole. The presence of cardiac chamber enlargement was established using sex-specific thresholds.15,16 The diagnostic performance of measurements from chest radiographs was evaluated with respect to detection of any cardiac chamber enlargement, defined as enlargement of at least 1 of the 4 cardiac chambers on MRI. Left ventricular enlargement was also evaluated separately, given that a few previous studies have evaluated the diagnostic performance of CTR with respect to left ventricular enlargement.10
Statistical analysis
We performed statistical analysis using Stata v14.1 (StataCorp). We considered a 2-tailed p value < 0.05 statistically significant. We tested continuous data for normal distribution using the Shapiro–Wilk test. We described continuous variables using means and standard deviations, and categorical variables using numbers and percentages. We evaluated the diagnostic performance of CTRs and cardiac diameter measurements using receiver operating characteristic curve analysis. We evaluated diagnostic performance of chest radiograph measurements in comparison with cardiac MRI, including sensitivity, specificity and positive and negative likelihood ratios. We assessed interobserver agreement using individual intraclass correlation coefficients with 2-way random effects models. We used linear regression to evaluate the association of enlargement of different cardiac chambers on MRI with maximum heart diameter and CTR on chest radiographs.
Ethics approval
This study was approved by the institutional research ethics board of the University Health Network, which waived the requirement for written informed consent.
Results
A total of 152 patients were included in the study, with a mean age of 52 (standard deviation [SD] 17) years (53% men) (Figure 2 and Table 1). The mean interval between chest radiography and MRI was 5.1 (SD 3.6) days. The prevalence of any cardiac chamber enlargement on MRI was 61%. Overall, 101 (66%) chest radiographs were PA radiographs and 51 (34%) were AP. We observed excellent interobserver reliability for all measurements from chest radiographs (Table 2).
Flow diagram of study participants. Note: MRI = magnetic resonance imaging.
Baseline clinical and imaging characteristics
Areas under the receiver operating characteristic curve for cardiac chamber enlargement and interobserver agreement
Heart diameter
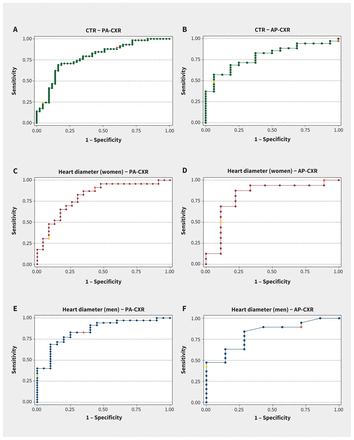
Among the measurements evaluated, maximum heart diameter (when calculated as a single measurement at any level) had the highest area under the receiver operating characteristic curve for detection of cardiac enlargement (Table 2). Maximum heart diameter was significantly higher in men than women (16.9 [SD 2.7] cm v. 15.1 [SD 2.3] cm, p < 0.001).
In the subgroup of PA chest radiographs, a maximum heart diameter cutpoint of 15 cm had a sensitivity of 86% and a negative likelihood ratio of 0.24, and a cutpoint of 19 cm had a specificity of 100% and a positive likelihood ratio of infinity among men (Table 3 and Figure 3). Among women, a maximum heart diameter cutpoint of 13 cm had a sensitivity of 91% and a negative likelihood ratio of 0.15, and a cutpoint of 17 cm had a specificity of 91% and a positive likelihood ratio of 3.5.
Sensitivity and specificity of specific cutpoints for detection of cardiac enlargement
Receiver operating characteristic (ROC) curves for measurements from patients with any cardiac chamber enlargement, including (A) cardiothoracic ratios (CTR) from posteroanterior chest radiographs (PA-CXR) and (B) anteroposterior chest radiographs (AP-CXR) for both men and women, (C) heart diameters measured from women with (C) PA-CXRs and (D) AP-CXRs, and for men with (E) PA-CXRs) and (F) AP-CXRs. Upper cutpoints are shown in yellow and lower cutpoints are shown in orange.
Maximum heart diameter was significantly higher in the subgroup with AP chest radiographs than those with PA chest radiographs (17.0 [SD 2.5] cm v. 15.6 [SD 2.7] cm, p = 0.002). In the AP subgroup, a maximum heart diameter cutpoint of 15 cm had a sensitivity of 90% and a negative likelihood ratio of 0.37, and a cutpoint of 19 cm had a specificity of 100% and a positive likelihood ratio of infinity among men. Among women, a maximum heart diameter cutpoint of 13 cm had a sensitivity of 100% and a negative likelihood ratio of 0, and a cutpoint of 17 cm had a specificity of 89% and a positive likelihood ratio of 4.5.
Cardiothoracic ratio
Among the 6 CTRs evaluated, the CTR assessed as the ratio of maximum heart diameter (from a single measurement) divided by maximum thoracic diameter (at any level) had the highest area under the curve and highest reproducibility, and was used for all subsequent analyses of CTR. The CTR did not differ significantly between men and women (0.53 [SD 0.08] v. 0.54 [SD 0.09], p = 0.2).
In the subgroup of PA chest radiographs, a CTR cutpoint of 0.50 had moderate sensitivity (72%) and specificity (72%), a positive likelihood ratio of 2.6 and a negative likelihood ratio of 0.38. However, a cutpoint of 0.45 had a sensitivity of 88% with a negative likelihood ratio of 0.31, and a cutpoint of 0.60 had a specificity of 95% and a positive likelihood ratio of 5.2.
The CTR was significantly higher in the subgroup of AP chest radiographs (0.57 [SD 0.08] v. 0.52 [SD 0.08], p = 0.002). In this subgroup, a traditional CTR cutpoint of 0.50 had a sensitivity of 89%, a specificity of 38%, a positive likelihood ratio of 1.40 and negative likelihood ratio of 0.31. However, a CTR cutpoint of 0.45 had a sensitivity of 97% with a negative likelihood ratio of infinity, and a cutpoint of 0.60 had a specificity of 94% and a positive likelihood ratio of 7.80.
Compared with a traditional CTR cutpoint of 0.5, a CTR cutpoint of 0.6 correctly reclassified 19 (32%) of 59 of patients without cardiac enlargement as negative, and a CTR cutpoint of 0.45 correctly reclassified 12 (13%) of 93 of patients with cardiac enlargement as positive.
Sensitivity analysis
We also evaluated the proposed measurement cutpoints for detection of cardiac chamber enlargement in subsets of chest radiographs with rotation and limited inspiration. In the subset with rotation, a CTR cutpoint of 0.45 had a sensitivity of 100%, and a cutpoint of 0.60 had a specificity of 91%. In men, a heart diameter cutpoint of 15 cm had a sensitivity of 100%, and a cutpoint of 19 cm had a specificity of 69%; in women, a heart diameter cutpoint of 13 cm had a sensitivity of 100%, and a cutpoint of 17 cm had a specificity of 79%. In the subset with limited inspiration, a CTR cutpoint of 0.45 had a sensitivity of 96%, and a cutpoint of 0.60 had a specificity of 94%. In men, a maximum heart diameter cutpoint of 15 cm had a sensitivity of 94%, and a cutpoint of 19 cm had a specificity of 100%; in women, a maximum heart diameter cutpoint of 13 cm had a sensitivity of 100%, and a cutpoint of 17 cm had a specificity of 81%.
We evaluated the same proposed measurement cutpoints for detection of left ventricular enlargement (Table 4). Accuracy was similar overall to the results for cardiac chamber enlargement, except that for left ventricular enlargement, sensitivities tended to be slightly higher and specificities tended to be slightly lower.
Sensitivity and specificity of specific cutpoints for detection of left ventricular enlargement
Contributions of cardiac chamber enlargement
Enlargement of each individual cardiac chamber was positively associated with both CTR and maximum heart diameter on linear regression (Table 5). For example, patients with enlargement of any cardiac chamber had a maximum heart diameter that was 2.97 cm larger, on average, than in patients with no cardiac chamber enlargement.
Linear regression parameters of the cardiothoracic ratio and maximum heart diameter on enlargement of different cardiac chambers
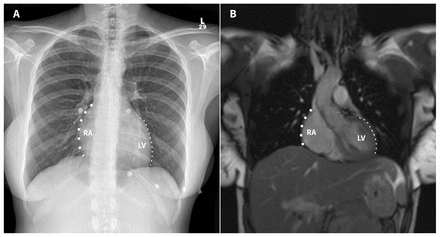
Right atrial enlargement had the highest R2 values, which indicates that variability in right atrial enlargement explains a higher proportion of variation in both maximum heart diameter and CTR than enlargement of the other cardiac chambers (Figure 4). Overall, enlargement of any cardiac chamber explained 23.0% of variation in CTR and 28.7% of variation in maximum heart diameter.
(A) Frontal posteroanterior chest radiograph and (B) coronal steady-state free precession cardiac magnetic resonance imaging scan of a 32-year-old woman, showing the relative contributions of cardiac chambers to the right and left cardiac borders on chest radiographs. The right heart border is formed primarily by the right atrium (RA, thick dotted line) and the left heart border is formed primarily by the left ventricle (LV, thin dotted line).
Interpretation
The results of this study show that a traditional CTR cutpoint of 0.5 has limited diagnostic utility, given moderate sensitivity and specificity. However, the simple heart diameter measurements and new CTR cutpoints proposed have higher diagnostic performance. A CTR cutpoint of 0.45 and a maximum heart diameter cutpoint of 13 cm in women and 15 cm in men have high sensitivity and low negative likelihood ratios, and are useful in ruling out cardiac chamber enlargement when negative. A CTR cutpoint of 0.60 and a maximum heart diameter cutpoint of 17 cm in women and 19 cm in men have high specificity and moderately high positive likelihood ratios, and are useful in ruling in cardiac chamber enlargement when positive. Cardiothoractic ratio values between 0.45 and 0.60 and heart diameter measurements between 13 and 17 cm in women and between 15 and 19 cm in men are indeterminate and are not helpful in ruling in or out cardiac enlargement. These findings were relatively robust on sensitivity analysis. Therefore, we have not proposed separate cutpoints for different scenarios (for example, on AP v. PA chest radiographs) to keep the suggested cutpoints simple and to facilitate ease of use in routine clinical practice.
Only a few previous studies have evaluated the diagnostic performance of CTR in detecting cardiac enlargement using 2-dimensional transthoracic echocardiography as the reference standard, with low specificity.17,18 The strengths of the current study include use of cardiac MRI as the reference standard, a relatively large sample of men and women, and sex-specific analysis for heart diameter measurements. Men tend to have slightly larger cardiac chamber sizes than women, and therefore we evaluated maximum heart diameter cutpoints by sex.15,19 Use of a single cutpoint for both sexes would result in lower specificity in men and lower sensitivity in women. On the other hand, CTR is calculated by dividing heart diameter by thoracic diameter as an approximate index of body size. Unlike maximum heart diameter, we found that CTR did not differ significantly between men and women and, therefore, we have not proposed sex-specific cutpoints for CTR.
We suggest using the proposed sex-specific measurement cutpoints for heart diameter when evaluating cardiac enlargement on chest radiographs in most scenarios, given that maximum heart diameter is easier to measure than calculating CTR and has higher diagnostic performance. However, CTR generally had slightly higher specificity than heart diameters in cases with limited inspiration and on AP chest radiographs. Therefore, CTR may be considered in these settings or when it is unclear if natal sex is the best predictor of cardiac size (e.g., for transgender people who started gender-affirming hormone treatment at a relatively young age).20 When evaluated, CTR should be assessed as the ratio of maximum heart diameter to maximum thoracic diameter, given that this technique has the best diagnostic performance and reproducibility. Danzer’s original calculations of the CTR divided the transverse diameter of the heart (obtained by adding the widest distance of the right and left heart borders to the midline) by the largest thoracic diameter. 8 We found that assessing the CTR using this technique had lower diagnostic performance and reproducibility than calculating the CTR as the ratio of the maximum heart diameter (as a single measurement) to maximum thoracic diameter. The original formula is also more cumbersome to calculate, given the need to sum the right and left heart measurements, and is therefore not recommended.
In general, the negative likelihood ratios for the lower cutpoints proposed in this study change the pretest probability of disease more substantially than the positive likelihood ratios for the upper cutpoints, suggesting that chest radiography may be more useful for ruling out cardiac chamber enlargement than for ruling it in. The lower cutpoints have high sensitivity, which is helpful to rule out disease with a high degree of confidence, thereby minimizing the number of false-negative results. For patients with moderate-to-low pretest probability of disease, we suggest using the lower sex-specific maximum heart diameter cutpoints proposed in this study. In this scenario, a negative result could lower the odds of cardiac enlargement sufficiently that clinicians can exclude this diagnosis and focus on other differential considerations.
On the other hand, the proposed upper cutpoints have high specificity, and may be helpful to rule in disease when positive. Minimizing false-positive results is important, given the cost, time and patient anxiety associated with downstream testing.21,22 However, the sensitivity was relatively low for the proposed upper cutpoints such that the positive likelihood ratios may be less useful in clinical practice. Given that chest radiographs are relatively inexpensive and are useful in investigating other potential disease processes (e.g., pulmonary edema), we suggest using the upper cutpoints for patients with moderate-to-high pretest likelihood of cardiac chamber enlargement who are undergoing chest radiographs for another indication. In this scenario, a positive result may increase the odds of cardiac enlargement sufficiently to warrant further confirmatory testing (e.g., echocardiography). It is important to highlight that the clinical relevance of the cutpoints proposed in this study will depend on the pretest probability of disease.
Enlargement of each individual cardiac chamber is positively associated with both CTR and maximum heart diameter. The right atrium typically forms the right heart border and the left ventricle typically forms the left heart border on frontal chest radiographs. Enlargement of the right atrium was the largest contributor to increased CTR and maximum heart diameter on chest radiographs, similar to the findings in a previous study.23 The slightly lower contribution of the left ventricle to heart diameter might be related to the rotation of the heart as it enlarges. Left ventricular dilation is associated with counter-clockwise rotation of the heart within the thorax, which can result in a normal CTR.24 On the other hand, enlargement of the right ventricle is associated with clockwise rotation of the heart, thus increasing the transverse diameter of the heart despite the fact that the right ventricle does not typically form one of the cardiac borders on frontal chest radiographs.
Limitations
We conducted our study at a single large tertiary referral centre and results may not be generalizable to all settings. Several potential pitfalls may render CTR and heart diameter measurements inaccurate for estimating cardiac enlargement, including an abnormal chest configuration, malposition of the mediastinal structures or vessels and presence of a pericardial effusion. Only patients who had undergone both chest radiography and cardiac MRI within a 14-day interval were included, which could result in selection and referral bias as this cohort of patients is expected to have a higher prevalence of cardiac enlargement than the general population of patients undergoing chest radiography. The prevalence of cardiac chamber enlargement in patients undergoing chest radiography has been reported as 7%, whereas in our study, the prevalence was 61%, as determined by MRI.25,26 Sensitivity and specificity are considered to be relatively robust to differences in prevalence, given that sensitivity is estimated in people with the disease and specificity is estimated in people without the disease.27 However, other analyses have suggested that specificity may be slightly lower with higher disease prevalence, with no systematic effect for sensitivity.28 The proposed measurement cutpoints in this study should be validated in a future independent study; for example, by recruiting a consecutive or random sample of patients referred for chest radiography, all of whom would undergo confirmatory diagnostic evaluation with MRI.
Conclusion
A traditional CTR cutpoint of 0.5 has limited diagnostic utility for detection of cardiac enlargement. Alternate CTR and sex-specific heart diameter cutpoints, proposed in this study, have reasonable diagnostic performance and may be particularly useful for ruling out cardiac enlargement when negative.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Kate Hanneman was responsible for study design. Felipe Torres, Diego Eifer and Felipe Sanchez Tijmes contributed to data collection, and all authors contributed to data analysis. Elsie Nguyen contributed to data interpretation. Kate Hanneman was responsible for manuscript writing and review. All authors contributed to manuscript revisions and review, gave final approval of the manuscript, and agree to be accountable for all aspects of the work.
Funding: No funding was received for this study.
Data sharing: Data are not available to others.
- Accepted September 2, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/