Abstract
Background: Emergency department visits and hospital admissions for opioid toxicity are opportunities to initiate opioid agonist therapy (OAT), which reduces morbidity and mortality in patients with opioid use disorder (OUD). The study objectives were to evaluate OAT initiation rates after a hospital encounter for opioid toxicity in Ontario, Canada, and determine whether publication of a 2018 Canadian OUD management guideline was associated with increased initiation.
Methods: We conducted a retrospective, population-based serial cross-sectional study of hospital encounters for opioid toxicity among patients with OUD between Jan. 1, 2013, and Mar. 31, 2020, in Ontario, Canada. The primary outcome was OAT initiation (methadone, buprenorphine–naloxone, or slow-release oral morphine) within 7 days of discharge, measured quarterly. We examined the impact of the release of the OUD management guideline on OAT initiation rates using Autoregressive Integrated Moving Average models.
Results: Among 20 702 hospital visits for opioid toxicity among patients with OUD, the median age was 35 years, and 65.1% were male. Over the study period, the percentage of visits leading to OAT initiation within 7 days rose from 1.7% or less (Q1 2013) to 5.6% (Q1 2020); however, the publication of the Canadian OUD management guideline was not associated with a significant increase in these rates (0.14% slope change, 95% confidence interval −0.11% to 0.38%; p = 0.3).
Interpretation: Among hospital encounters for opioid toxicity, despite rising prevalence over time, only 1 in 18 patients were dispensed OAT within a week of discharge in early 2020. These findings highlight missed opportunities to initiate therapies proven to reduce mortality in patients with OUD.
Opioid use disorder (OUD) is a substantial public health problem with an increasing prevalence worldwide.1 From 2016 to 2021, 26 690 opioid-related deaths occurred in Canada;1 in Ontario, two-thirds of these deaths were among patients with OUD,2 and 1 in 219 Ontarians was treated for OUD in 2021.3 In Canada, opioid-related hospital admissions increased by 32% between 2016 and 2021,4 and in Ontario, opioid-related emergency department visits increased by 286%.5 Among patients presenting to the emergency department with nonfatal opioid overdose, close to 5% die within 1 year.6,7
Opioid agonist therapy (OAT) is a highly effective approach to reducing morbidity and all-cause mortality in patients with OUD.8,9 Patients are more likely to continue treatment if OAT is started in the emergency department than if they are referred for outpatient treatment.10 A randomized controlled trial in the United States showed that compared with brief intervention or outpatient referral, buprenorphine–naloxone treatment initiated in the emergency department increased treatment engagement and decreased unregulated drug use.10 Despite strong evidence supporting the use of OAT in reducing morbidity and mortality, 8,9,11 only a small minority of patients who survive an opioid overdose receive OAT in the US, with numbers ranging from 5% to 8.5% in the 30 days after initial visit.12,13 Because studies have shown the mortality risk to be highest in the days immediately after an overdose,6 timely OAT initiation and care are essential.
A 2018 Canadian clinical practice guideline was the first national guideline to recommend buprenorphine–naloxone as first-line treatment to reduce the risk of toxicity and facilitate safer take-home dosing.9 Methadone is recommended as second-line treatment when patients respond poorly to buprenorphine–naloxone or it is not the preferred option for another reason.9 Slow-release oral morphine can be considered as third-line treatment when buprenorphine–naloxone and methadone are ineffective or contraindicated yet patients remain at high risk of opioid-related harms.9
As rates of hospital-treated opioid toxicity continue to rise, there is a need to understand OAT initiation patterns in this population. Therefore, we sought to examine trends in OAT initiation rates for patients presenting to hospital with acute opioid toxicity, determine whether the 2018 clinical guideline was associated with increased OAT initiation, and describe characteristics of OAT prescribers.
Methods
Study design and setting
We conducted a population-based serial cross-sectional study to examine the rate of initiation of methadone, buprenorphine–naloxone, or daily dispensed slow-release oral morphine within 7 days after an emergency department visit or hospital admission for opioid toxicity among residents of Ontario, Canada, between Jan. 1, 2013, and Mar. 31, 2020. We selected this study period based on data availability and the onset of the COVID-19 pandemic, which we expected to greatly affect patterns of hospital-and community-based care. We have reported this study following the Reporting of Studies Conducted using Observational Routinely Collected Data (RECORD) guideline.
Data sources
We used data housed at ICES, an independent, nonprofit research institute in Ontario, Canada, with legal status that allows for the collection and analysis of administrative health care and demographic data without consent, for health system evaluation and improvement. We used the Canadian Institute for Health Information Discharge Abstract Database, National Ambulatory Care Reporting System and Ontario Mental Health Reporting System to determine diagnoses occurring during hospital admissions and emergency department visits. We used the Narcotics Monitoring System (available from July 2012 onward) to capture outpatient dispensing of OAT, regardless of payer. We used the Ontario Health Insurance Plan (OHIP) Registered Persons Database to determine demographic variables and the OHIP Claims database and Ontario Drug Benefit database to determine clinical diagnoses and health services utilization. These data sets were linked using unique encoded identifiers and analyzed at ICES.
Identification of the cohort
We constructed a cohort of patients who visited the emergency department or were admitted to an acute-care hospital for opioid toxicity between Jan. 1, 2013, and Mar. 31, 2020. We defined opioid toxicity events using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, diagnosis codes T40.0 to T40.4 or T40.6, which have been used in previous studies (see Appendix 1, eTable 1 for descriptions, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231014/tab-related-content).7,14 We defined the index date as the emergency department or hospital discharge date for the opioid toxicity event. We excluded patients who were transferred to other acute or nonacute care hospitals after discharge, were not Ontario residents, were younger than 15 years, were dispensed OAT in the preceding 30 days (to remove patients in treatment at time of hospital admission), died in hospital, or had no OUD documented history (Appendix 1, eTable 2; defined as having a hospital encounter or outpatient physician visit with a diagnosis of OUD, or being prescribed OAT in the 5 years before). This final exclusion was applied to ensure that those experiencing the opioid toxicity had an OUD diagnosis and therefore were eligible to initiate treatment. In cases where a patient experienced multiple opioid toxicity events that met the inclusion criteria over the study period, all events were included.
Outcomes
Our primary outcome was community-based initiation of OAT within 0–7 days of discharge from the emergency department or hospital after an opioid toxicity event. We defined community-based initiation as a new prescription filled for methadone, buprenorphine–naloxone, or slow-release oral morphine from a community-based pharmacy. To exclude patients who received slow-release oral morphine for pain rather than for OAT, we included only those who were initiated on daily dispensed therapy, which is the practice recommended by the OUD management guideline.9 Our secondary outcome was any health care visit (outpatient physician visit, emergency department visit or hospital admission for any cause) in the 1–7 days after the index date, to identify opportunities for additional connection to treatment and supports related to substance use soon after the toxicity event. In a sensitivity analysis, we replicated our primary outcome, considering only those OAT initiations that had occurred on the date of discharge or the day thereafter.
Statistical analysis
We calculated descriptive statistics for sociodemographic characteristics (age, sex, urban or rural residence location, neighbourhood income quintile, Ontario Marginalization Index; comorbidities measured using the Johns Hopkins ACG System Version 10 Aggregated Diagnosis Groups, mental health diagnoses, substance use disorders, diagnosis of infective endocarditis, HIV and hepatitis C); previous health-service use (hospital admission or emergency department visit for opioid toxicity in the past year, previous OAT in the past year, and number of physician visits, emergency department visits or hospital admissions in the 1 year before the index date), and index hospital visit characteristics (length of hospital admission, admission to an intensive care unit and discharge disposition). We stratified these characteristics according to whether OAT was initiated within 7 days of the index date. We used absolute standardized differences (SDs) to test for meaningful differences between groups, with a value greater than 0.10 suggesting imbalance.15 Among patients initiating OAT, we described the characteristics of the OAT prescriber, including age, sex, years in practice and medical specialty.
We created a quarterly time series from Jan. 1, 2013, to Mar. 31, 2020, and calculated a Cochran–Armitage test to assess for trends over time. We used Autoregressive Integrated Moving Average models to examine whether the introduction of the national OUD management guideline in March 2018 was associated with an increase in OAT initiation rates. We hypothesized that any change would be gradual and therefore modelled the intervention using a ramp function, which tests for a change in slope at the time of the guideline’s release.16 We assessed stationarity using augmented Dickey–Fuller tests and differenced the time series as needed to produce a stationary time series. We examined the autocorrelation function, partial autocorrelation function and inverse correlation function plots to determine the appropriate moving average or autoregressive terms for the models. We assessed the fit of the models using residual autocorrelation, partial autocorrelation and inverse correlation function plots. We conducted a sensitivity analysis modelling monthly data to assess the robustness of our findings. However, quarterly data are reported to prevent disclosure of counts of 5 or less, in accordance with institutional privacy policy. All analyses were conducted at ICES using SAS Enterprise Guide 7.1.
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which did not require review by a Research Ethics Board or informed consent.
Results
Among 47 910 emergency department visits or hospital admissions for opioid toxicity over the study period, 20 702 (43.2%) events among 14 053 patients met our inclusion criteria. Most patients had only 1 toxicity event contributing to the analysis (n = 10 609; 75.5%). The primary reasons for exclusion were recent receipt of OAT (n = 8559; 17.9%) and no documented OUD diagnosis in the preceding 5 years (n = 11 896; 24.8%) (Figure 1). The median age was 35 years (interquartile range [IQR] 27–48 years), 65.1% (n = 13 475) were male, and 89.9% (n = 18 614) resided in urban areas (Table 1). The neighbourhood income distribution was skewed, with 40.0% in the lowest quintile. In terms of previous health services use, 28.6% (n = 5912) of events occurred among patients with a previous emergency department visit or hospital admission for opioid toxicity, and 24.1% (n = 4989) had been dispensed OAT in the last year.
Inclusion and exclusion criteria applied to population-based analysis to create a cohort of patients with opioid use disorder with an emergency department (ED) visit or hospital admission for opioid toxicity. Index date refers to first emergency department visit or hospital admission for opioid toxicity. Note: SROM = slow-release oral morphine.
Baseline demographic and clinical characteristics of patients, overall and by opioid agonist therapy initiation within 7 days of discharge from index emergency department visit or hospital admission for an opioid toxicity event
Over the entire study period, 851 events (4.1%) led to OAT initiation within 7 days of discharge from the emergency department or hospital. About one-third (36.1%) of OAT initiations occurred on the date of hospital discharge or the following day, with 48.1% occurring on days 2–5, and only 15.9% occurring on days 6 or 7 (Appendix 1, eTable 3). Compared with patients who experienced events that did not lead to OAT initiation, those who initiated were younger (median 32 yr v. 35 yr; SD 0.23), and more likely to live in the highest neighbourhood income quintile (13.6% v. 9.6%; SD 0.12), have had an episode of opioid toxicity in the preceding year (35.5% v. 28.3%; SD 0.16), and have been prescribed OAT in the previous year but not within 30 days of the index visit (53.5% v. 22.8%; SD 0.66).
Among 5219 inpatient hospital admissions for opioid toxicity, 215 (4.1%) initiated OAT within 7 days of discharge (Table 2). Hospital stays that led to OAT initiation were more likely to be longer (median 3 d v. 2 d; SD 0.26) and involve an intensive care admission (51.2% v. 45.7%; SD 0.11). Among the 15 483 opioid toxicities treated exclusively in an emergency department, 636 (4.1%) led to OAT initiation within 7 days of discharge (Table 2). In total, 15.3% of the cohort in hospital and 14.2% of the cohort in the emergency department were patient-initiated discharges; however, this did not differ between OAT initiation groups in either setting.
Characteristics of hospital admissions and emergency department visits for opioid toxicity events, overall and by opioid agonist therapy initiation within 7 days of discharge*
We identified 379 OAT prescribers in the study cohort, of whom 352 (92.9%) could be linked with provider-level data (Table 3). The median age of prescribers was 46 years (IQR 37–56 yr), and 70.2% (n = 247) were male. Most prescribers (n = 260; 73.9%) had been practising medicine for 10 or more years and were general practitioners (n = 238; 67.6%).
Demographics and characteristics of opioid agonist therapy prescribers
In the secondary analysis of health care encounters, we found that in the 7 days after hospital discharge for opioid toxicity, 22.1% of patients (n = 4573) had an outpatient visit, 17.8% (n = 3675) of patients visited the emergency department and 3.1% (n = 647) were admitted to hospital (Table 4). This differed between OAT initiation groups, with those starting OAT in the first week being more likely to have an outpatient visit (75.6% v. 19.8%; SD 1.35) and emergency department visit (23.6% v. 17.5%; SD 0.15) compared with those not starting OAT; however, there was no significant difference in the rate of hospital admissions between OAT initiation groups (2.9% v. 3.1%; SD 0.01).
Health care encounters in the 7 days after discharge from emergency department or inpatient hospital admission
Impact of the Canadian OUD clinical management guideline
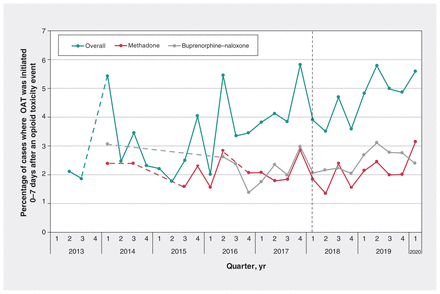
The OAT initiation rate after opioid toxicity increased from 1.7% or less (n ≤ 5) in the first quarter of 2013, to 5.6% in the first quarter of 2020 (Figure 2). Graphical examination suggested considerable fluctuation throughout the observation period, but the OAT initiation rate began to gradually increase in 2016, which is reflected by a statistically significant increasing trend over time in OAT initiation rates (p < 0.0001). Trends were generally consistent when stratifying by OAT type. The methadone initiation rate rose from 1.7% or less to 3.1% over the study period (Q1 2013 v. Q1 2020) and the buprenorphine–naloxone initiation rate rose from 1.7% or less to 2.4%. Only 19 patients initiated slow-release oral morphine after a toxicity event. In the sensitivity analysis considering OAT initiation rates on the date of discharge or following day, our findings were generally consistent with the primary analysis, with increases over time, reaching 2.0% in Q1 2020 (Appendix 1, eFigure 1). In the quarterly time series analysis (see model specifications in Appendix 1, eTable 4), we found no significant impact of the introduction of the national guideline for OUD management on the 7-day OAT initiation rate after an opioid toxicity event overall (0.14% slope change, 95% confidence interval [CI] −0.11% to 0.38%; p = 0.3) or by OAT type (methadone: 0.07% slope change, 95% CI −0.12% to 0.25%; p = 0.5; and buprenorphine–naloxone 0.04% slope change, 95% CI −0.26% to 0.34%; p = 0.8; Appendix 1, eTable 4). In the sensitivity analysis using monthly data (data not shown owing to institutional policies that preclude the publication of small cell sizes), the results were consistent (p = 0.6 overall, p = 0.6 for methadone and p = 0.8 for buprenorphine–naloxone; Appendix 1, eTable 4).
Rate of opioid agonist therapy (OAT) initiation 0–7 days after an opioid toxicity event resulting in hospital admission or emergency department visit. Note: There were 19 cases of initiation of daily dispensed slow-release morphine from 2013 to 2020. These are included in the overall data in this figure, but are not reported separately owing to the small number of patients. Quarterly data are reported to prevent disclosure of counts ≤ 5 wherever possible. Dashed lines are used to connect data points where event counts ≤ 5 have led to suppression of quarterly data. The Canadian guideline recommending OAT9 was released in March 2018. We noted no significant change on OAT initiation rates after the guideline was released (overall p = 0.3, methadone p = 0.5, buprenorphine–naloxone p = 0.8).
Interpretation
In this population-based study of more than 20 000 emergency department visits or hospital admissions for opioid toxicity among patients with OUD without recent treatment, the OAT initiation rate within a week of discharge was low, reaching only 5.6% by the first quarter of 2020. Our research shows that there were substantial disparities in OAT initiation rates, with potential barriers to prescribing for older patients, those with mental health diagnoses and those in the lowest neighbourhood income quintile. Although OAT initiation rates have gradually increased since 2016, the release of the national OUD management guideline in 2018 was not independently associated with changes in this trajectory.
Our study examined temporal trends in OAT initiation in the first 7 days after discharge, a time frame associated with high mortality risk, and showed that only 4.1% of hospital encounters for opioid toxicity led to treatment initiation during the study period. Similarly, a study in the US reported that only 3.8% of emergency department patients presenting with an opioid overdose received buprenorphine within 7 days.12 However, we also found that 22.1% of opioid toxicity events led to an outpatient visit within 7 days after hospital discharge for opioid toxicity despite the low OAT initiation rate. These results highlight critical missed opportunities to prevent future mortality and morbidity related to opioid use, despite connection to health care for many patients in the days after a toxicity event.
A US study showed that only 20.9% of emergency department physicians felt ready to prescribe buprenorphine–naloxone, citing a lack of formal training, limited knowledge of resources and absence of local protocols and referral networks.17 Other research similarly suggests that barriers to OAT initiated in the emergency department include a shortage of trained prescribers and limited knowledge of outpatient follow-up options;18 fewer than half of emergency department clinicians felt comfortable initiating methadone. 19 More research is needed to further elucidate the reasons for low OAT prescribing within hospital settings in Ontario, but effective responses may include providing institutional OAT training, establishing OAT initiation protocols in acute care settings, organizing and promoting awareness of referral networks with outpatient addictions programs,20 and improving prescribing for patient-initiated discharges.
Limitations
This study had several limitations. First, we used administrative health data, which contain limited information on in-hospital medication provision and discharge prescriptions. We were thus unable to determine whether a discharge prescription for OAT was provided in the hospital setting or whether OAT was initiated in the hospital. We therefore defined our outcome as community-based initiation of OAT, recognizing that this will include patients who continued OAT that was initiated during their hospital stay, and those who were referred to treatment and initiated within the community soon after discharge. We are also unable to identify prescriptions for OAT that were provided in a hospital setting at discharge but never filled. Therefore, it is possible that the low prevalence of OAT initiation in our study is influenced by both underprescribing and poor access and connection to community-based OAT for patients with OUD who were considering initiating treatment. Further, the decision to initiate OAT can be complex, taking into account many factors (including patient preference, possible negative experiences with OAT or health care systems and difficulties meeting needs of patients with high opioid tolerances with first-line OAT), which cannot be captured with administrative data.
Second, there is no validated OUD definition, and we had to rely on hospital or physician visits with an OUD diagnosis or previous OAT to define this diagnosis. This can misclassify patients with OUD who are not engaged in the health care system and those whose health care interactions do not adequately reflect their OUD.
Third, we were not able to determine which opioid or combination of substances led to the overdose, which could influence the severity of the toxicity and likelihood of treatment engagement.
Finally, we excluded those patients who were dispensed OAT in the 30 days before hospital admission, to allow us to identify new OAT recipients. However, this could exclude some patients who had recently discontinued OAT and experienced a toxicity event, who might have been more likely to be reinitiated into treatment.
Conclusion
Despite slight increases over time, OAT initiation rates after an emergency department visit or hospital admission for opioid toxicity in Ontario were low, with only 1 in 18 events leading to filling an OAT prescription within a week of discharge. The release of a national guideline advocating for buprenorphine–naloxone as first-line therapy did not appear to substantially influence OAT initiation rates, suggesting that additional efforts are needed to improve initiation of OAT in acute care settings.
Footnotes
Competing interests: David Juurlink reports serving as the principal investigator on a grant from PSI Foundation to study the use of buprenorphine after nonfatal overdose. Dr. Juurlink has received payment for expert testimony from multiple law firms related to individual actions involving opioids, outside the submitted work. Dr. Juurlink is also a volunteer member of Physicians for Responsible Opioid Prescribing (a US organization that advocates for opioid stewardship). Dr. Juurlink holds the view that buprenorphine is an invaluable therapy with a safety profile that is far superior to full opioid agonists; some have perceived this as an intellectual competing interest. Ahmed Bayoumi reports receiving grants from the Canadian Institutes for Health Research in support of this study (paid to institution), and from the Baxter and Alma Ricard Chair in Inner City Health, outside of the submitted work. Dr. Bayoumi has also received consulting fees from the Canadian Centre for Substance Abuse. Pamela Reece reports employment with Public Health Ontario (Ontario for Health Protection and Promotion), as courtesy staff at the Women’s College Hospital, and as a physician at the Kickstart Medical Clinic. Tara Gomes reports receiving a grant from the Canadian Institutes for Health Research in support of this study (paid to institution). Dr. Gomes has also received a grant and contract funding from the Ontario Ministry of Health and Ontario College of Pharmacists to support a research program (paid to institution), outside the submitted work. Dr. Gomes is supported by a Tier 2 Canada Research Chair in Drug Policy Research and Evaluation, which supports salary. Dr. Gomes has also personally received contract funding from the Canadian Agency for Drugs and Technologies in Health for roles as expert reviewer on projects and grant reviewer; fees for consulting on an audit for the British Columbia Auditor General; and support from Indigenous Services Canada for participation on the Drugs and Therapeutics Advisory Committee. Dr. Gomes has also served as a member of the Board of Governors for the Inner City Family Health Team. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All authors contributed to the conception and design of the work, and the acquisition, analysis, and interpretation of data for the work. Tina Hu drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding: This study was funded by a Canadian Institutes for Health Research grant (grant numbers 153070 and 165949). This study was also supported by ICES. Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health and Canadian Institute for Health Information. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. This document used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. The authors thank IQVIA Solutions Canada Inc. for use of their Drug Information File and the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Data sharing: The data set from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., health care organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das{at}ices.on.ca).
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
- Accepted October 11, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/













Podcast