Chronic hyperkalemia is prevalent among patients with chronic kidney disease, heart failure or other conditions treated with renin–angiotensin–aldosterone system (RAAS) inhibitors, and has been associated with severe adverse outcomes, including death.
The risk of chronic hyperkalemia may be mitigated by reducing dietary potassium, careful dosing of hyperkalemia-inducing medications, using diuretics that promote renal extraction of hyperkalemia and using potassium binders.
Accumulating evidence suggests that the adverse effects of reducing or stopping RAAS inhibitors outweigh the potential harms of hyperkalemia, and guidelines recommend maintaining optimal RAAS inhibitor dosing by treating hyperkalemia using other strategies.
More research is needed to support the prevention and management of hyperkalemia among patients with chronic kidney disease or heart failure.
Hyperkalemia, defined as a serum potassium level of 5.0 mmol/L or greater, can lead to severe electrophysiological disturbances, including cardiac arrythmias, that increase morbidity and risk of death.1 It is common in patients with conditions that impair potassium excretion by the kidneys, such as chronic kidney disease (CKD), heart failure, hypertension that is difficult to control, diabetes or combinations of these conditions.1,2 These patients are commonly treated with renin–angiotensin–aldosterone system (RAAS) inhibitors to help lower their risk of CKD progression and cardiovascular events.3 However, these medications may trigger or exacerbate hyperkalemia.4–7 Some guidelines now recommend initiating and up-titrating these medications to the highest approved dose that the patient can tolerate to optimize clinical outcomes.8,9 Therefore, anticipation and prompt management of hyperkalemia is crucial for patients prescribed these medications. We discuss strategies to mitigate the risk of chronic hyperkalemia and to optimize care of patients being treated for CKD, heart failure or associated conditions, as informed by original research, reviews and clinical practice guidelines (Box 1).
Evidence used in this review
We conducted a targeted search of MEDLINE to identify original research and review articles on hyperkalemia published through February 2021. Medical subject heading search terms included “hyperkalemia,” “chronic kidney disease,” “heart failure” and “management.” In addition, we reviewed current European, American and Canadian guidelines on the management of hyperkalemia.
Who is at risk of hyperkalemia?
Conditions that interfere with the kidney’s ability to excrete potassium contribute to the development of hyperkalemia because potassium homeostasis is largely achieved by renal excretion. A population-based cohort study found that 28% of patients with CKD and 39% of patients with heart failure had at least 1 episode of hyperkalemia.10 However, the prevalence of hyperkalemia, as well as the risk of recurrence, increases with a higher burden of comorbid conditions.2,11
Other important risk factors include advanced age and the use of medications known to increase serum potassium levels.4 Among the medications that lead to hyperkalemia, the most clinically important are RAAS inhibitors, which include angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers and mineralocorticoid receptor antagonists.4–6 Although there is heterogeneity among studies, a systematic review and meta-analysis of 20 studies found that hyperkalemia is nearly twice as common (risk ratio 1.89, 95% confidence interval 1.56–2.30) in patients on drugs that act on the RAAS.5 Moreover, data from clinical trials have also shown an increase in the incidence of hyperkalemia in patients with CKD or heart failure on RAAS inhibitor therapy.12
An episode of hyperkalemia puts patients at risk of a second episode. An analysis of population-based registries found that second episodes of hyperkalemia were documented within 6 months of the initial occurrence in 40% of patients with CKD, 49% of patients with heart failure, and 37% of patients using RAAS inhibitors.10 Predictors of recurrence included a moderate-to-severe first episode of hyperkalemia (potassium ≥ 5.6 mmol/L), low estimated glomerular filtration rate (GFR), diabetes and use of spironolactone.10
What are the consequences of hyperkalemia?
Although it is well established that severe hyperkalemia is associated with an increased risk of death, even mild increases in potassium that are often deemed nonemergent may be of clinical importance. There is a “u-shaped” association between serum potassium levels and risk of death, with lowest risk of all-cause death in patients with potassium levels between 4.0 and less than 5.0 mmol/L.11 A retrospective cohort study from Manitoba found that, compared with patients with normal potassium levels, those with hyperkalemia (potassium ≥ 5.0 mmol/L) were at increased risk of all-cause death (hazard ratio [HR] 1.15, 95% confidence interval [CI] 1.13–1.18), cardiovascular events (HR 1.20, 95% CI 1.14–1.26) and death within 30 days (odds ratio [OR] 1.29, 95% CI 1.24–1.34).13
In Canada, emergency department use is high among patients with CKD. Observational studies have associated hyperkalemia with increased hospital (OR 1.71, 95% CI 1.68–1.74) and intensive care unit admissions (OR 3.48, 95% CI 3.34–3.62), and have found that hyperkalemia accounts for 48% of all ambulatory care–sensitive conditions among patients on dialysis.13,14 Therefore, unsurprisingly, hyperkalemia is associated with substantial costs from frequent hospitalizations for recurrent episodes.13,15
Physicians frequently respond to an episode of hyperkalemia by reducing or stopping a patient’s RAAS inhibitor regimen.14,16,17 However, submaximal dosing of RAAS inhibitors in patients with CKD or heart failure is associated with increased outpatient office visits and overall health care costs, and worse cardiorenal outcomes.16,18 This highlights the challenge of attempting to balance the cardiorenal benefits of RAAS inhibitor treatment with the associated risk of chronic hyperkalemia in patients who will benefit most from these drugs.
How often should clinicians assess patients’ potassium levels?
Patients at risk of chronic hyperkalemia may be asymptomatic, and frequent monitoring will increase detection of the condition.19 However, since many factors influence the risk of developing hyperkalemia, clinicians should consider several factors when deciding how frequently to test, particularly when initiating treatment with RAAS inhibitors (Box 2). Moreover, practices regarding the appropriate level of hyperkalemia at which to treat are not well standardized. Thresholds for treatment outlined in current guidance are based on averages of patients in a given laboratory system and the “u-shaped” association between serum potassium levels and risk of death.11
Suggested frequency of potassium monitoring8,19,20
Patients being considered for treatment with angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers:
At treatment initiation
1–2 weeks after initiation
1–4 weeks after final dose titration
At regular intervals thereafter based on the patient’s individual risk
Patients being considered for treatment with mineralocorticoid receptor antagonists:
1 and 2 weeks after initiating or increasing dose
At 8 and 12 weeks after initiation or increasing dose
At 6, 9, and 12 months after initiation or increasing dose
At regular intervals thereafter based on the patient’s individual risk
A rapid rise in potassium is more likely to lead to cardiac arrhythmias than a gradual increase so, when assessing a new result, it is important to consider how quickly the hyperkalemia has developed.21
Hyperkalemia in people with a preserved GFR is uncommon and is generally associated with pseudohyperkalemia, in which in vitro hemolysis leads to elevated potassium levels. Repeat testing to rule out pseudohyperkalemia should be considered unless the benefits of verification are outweighed by the potential harm of delaying treatment.19
Patient education, nursing support, follow-up (in person or by telephone), self-monitoring or point-of-care testing may facilitate regular monitoring and help ensure timely and effective treatment.20
How to manage patients at risk of hyperkalemia
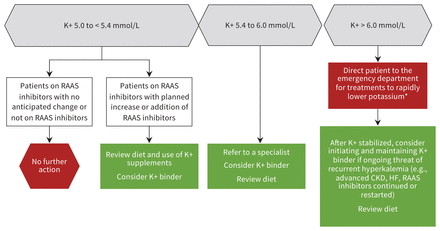
Figure 1 outlines an approach to the management of patients with hyperkalemia, which can be undertaken in the outpatient setting if resources allow. Beyond emergency treatment of hyperkalemia, experts suggest preventive approaches, such as encouraging patients to eat a low-potassium diet and to stop using supplements containing potassium. Other approaches include treating hypertension associated with heart failure with loop or thiazide diuretics, carefully dosing RAAS inhibitors and any other medications that may elevate potassium to balance potential benefits and risks, correcting concurrent acidosis, and using potassium binders.19
Strategies to mitigate the risk of hyperkalemia and enable more optimal care. Where applicable, clinicians should correct metabolic acidosis with oral sodium bicarbonate (if not contraindicated), promote potassium loss with diuretics in patients with volume overload (if not contraindicated) and consider the use of potassium binders, if available. *Although select patients with potassium values minimally elevated above 6.0 mmol/L can be managed as outpatients, given the variable availability of resources to rapidly treat and remeasure potassium in a timely fashion, management in the emergency department is recommended. Details of acute management are beyond the scope of this review. Note: CKD = chronic kidney disease, HF = heart failure, K+ = potassium, RAAS = renin–angiotensin–aldosterone system.
Reducing dietary potassium
Although guidelines recommend that patients at risk of hyperkalemia, in particular those with advanced CKD, decrease the amount of potassium in their diet, few trials have directly studied the impact of dietary potassium restriction in patients with CKD.9,19,22 Nonetheless, given the increased risk of chronic hyperkalemia for patients with CKD and heart failure, clinicians should consider referring these patients to a dietitian for guidance. The potential benefit of consuming a low-potassium, predominantly plant-based diet is an important area for future study.
Judicious use of diuretics
Diuretics, especially loop diuretics, are commonly used to control volume overload and prevent increases in serum potassium. The efficacy of diuretics to reduce serum potassium depends on a patient’s kidney function; guidelines recommend this intervention when diuresis or an additional antihypertensive agent is desired.19 Use of diuretics can precipitate acute kidney injury and electrolyte abnormalities, however, and the hypokalemic response to diuretics is diminished with low estimated GFR.3,8 Diuretics may not be ideal for the long-term maintenance of serum potassium levels, especially for patients with no other compelling reason for their use (i.e., extracellular volume overload).
Use of sodium bicarbonate
Treatment with oral sodium bicarbonate promotes potassium elimination through increased urinary potassium excretion. It can be used effectively to reduce the risk of hyperkalemia in patients with CKD and metabolic acidosis. However, in patients who develop volume overload, sodium bicarbonate may need to be stopped.8,19
Careful dosing of RAAS inhibitors
Reducing and stopping medications that induce hyperkalemia — such as RAAS inhibitors — are commonly used strategies for preventing recurrent episodes of hyperkalemia.3,19,22 However, given evidence that the benefits of RAAS inhibitors outweigh their potential harms, including hyperkalemia, recent guidelines now recommend that clinicians maintain an evidence-based dose of RAAS inhibitors. Lowering the dose to reduce the likelihood of recurrent hyperkalemia should be undertaken as a last resort after attempting measures to maintain the evidence-based dose of RAAS inhibitors, including using potassium binders.
Use of potassium binders
Sodium polystyrene sulfonate, sodium zirconium cyclosilicate and patiromer are potassium binders approved for use in Canada.8,9,23–25 For many years, sodium polystyrene sulfonate was the only medication specifically indicated for the treatment of hyperkalemia, but it was approved before the introduction of modern regulatory standards, and evidence from randomized trials to support its use is sparse.3 A small, single-centre study of 33 outpatients with CKD and mild hyperkalemia (5.0–5.9 mmol/L) found that sodium polystyrene sulfonate was superior to placebo in the reduction of serum potassium levels at 1 week (mean difference between groups −1.04 mmol/L, 95% CI, −1.37 to −0.71 mmol/L).26 However, the effects of the drug are not predictable and it has been associated with risk of acute bowel necrosis, hypernatremia, diarrhea and gastrointestinal toxicity, particularly with older formulations that contain sorbitol.27 One retrospective, matched cohort study found that, over 30 days, use of sodium polystyrene sulfonate was associated with nearly double the incidence of adverse gastrointestinal events than nonuse (37 events [0.2%] v. 18 events [0.1%], HR 1.94, 95% CI 1.10–3.41).28 Moreover, systemic alkalosis has been reported after administration of sodium polystyrene sulfonate in combination with nonabsorbable cation-donating antacids and laxatives such as magnesium hydroxide and aluminum carbonate; magnesium-containing laxatives should not be used with sodium polystyrene sulfonate.23 Thus, clinicians must be cognizant of the potential for serious adverse events when managing hyperkalemia with sodium polystyrene sulfonate. Newer potassium binders should be considered instead, if available.
Sodium zirconium cyclosilicate and patiromer, are newer potassium binders that are generally safer, more predictable and better tolerated by the patient (Table 1). Sodium zirconium cyclosilicate is an orally administered, insoluble, inorganic cation exchanger that selectively captures potassium ions in exchange for hydrogen and sodium ions in the gastrointestinal lumen, thereby increasing potassium fecal excretion and reducing serum potassium. Its efficacy and safety have been shown in phase 2 and 3 clinical trials in both patients receiving dialysis and those not on dialysis. Reported adverse events are generally mild to moderate in severity and are manageable without interruption of treatment. Edema has been reported, possibly a consequence of the drug containing sodium, and patients should be monitored for fluid overload.30–36 In clinical trials, 4.1% of patients treated with sodium zirconium cyclosilicate also developed hypokalemia with a serum potassium value less than 3.5 mmol/L, which was resolved by adjusting the dose or stopping the drug.25
Overview of potassium binders
Volume overload was not identified as an adverse outcome in trials of patiromer. This drug contains calcium as its gastrointestinal exchange ion and acts in the colon, which means its onset of action is relatively prolonged. The utility of patiromer in treating hyperkalemia has been shown in phase 2 and 3 trials of patients with hyperkalemia, including those with CKD and heart failure on RAAS inhibitors. No serious adverse events have been associated with patiromer, although reported adverse events include non-serious constipation, diarrhea, nausea or vomiting, abdominal discomfort, flatulence and electrolyte disturbances (e.g., hypokalemia and hypomagnesemia).37–40
A growing body of evidence supports using the newer potassium binders to facilitate the continuation and optimization of RAAS inhibitors in patients with hypertension, heart failure and CKD.35,37,38,40 In a phase 2, double-blind, placebo-controlled trial of patients with heart failure (PEARL-HF), patients randomized to patiromer showed a significantly lower incidence of hyperkalemia (7.3% v. 24.5% on placebo), which enabled greater use of spironolactone at 50 mg/d (91% v. 74%).37 Similarly, in the AMBER trial of patients with resistant hypertension and CKD, more patients in the treatment arm were able to continue spironolactone with less incidence of hyperkalemia than those on placebo.40 Although no study has been specifically designed to assess the RAAS inhibitor–enabling potential of sodium zirconium cyclosilicate, most patients with hyperkalemia enrolled in the ZS-005 study achieved normal potassium levels without substantial changes in their RAAS inhibitor regimen.35
Cost and drug plan reimbursement may limit adoption of the new potassium binders. Although the new potassium binders are funded through certain private plans, and the Canadian Agency for Drugs and Technologies in Health has recommended reimbursement of patiromer by public drug plans, it costs about $3500 to $7000 per patient annually, depending on dose.29 Further price negotiations are believed to be underway through the pan-Canadian Pharmaceutical Alliance. Manufacturers and drug plans are urged to find a price that allows access to these drugs for all patients who would benefit from them.
Conclusion
Hyperkalemia can have serious clinical consequences, including adverse cardiovascular outcomes and death.11 Hyperkalemia is associated with RAAS inhibitor therapy, particularly in patients with comorbidities such as CKD and heart failure.1 Reduction of RAAS inhibitor dosing may reduce potassium levels, but can have important consequences for optimal disease treatment.16,18 Although questions remain on their ability to improve outcomes, treatment with new potassium binders may mitigate the risk of recurrent hyperkalemia by rapidly and sustainably reducing serum potassium levels and enabling more optimal care of patients with guideline-recommended doses of RAAS inhibitors (Box 3).
Unanswered questions
What are the thresholds of mild, moderate and severe hyperkalemia, and at what threshold should treatment for chronic hyperkalemia be initiated?
What should be the standard method and frequency for determining potassium levels, and using what reference range?
Is dietary potassium restriction effective for patients with chronic kidney disease or heart failure who are at risk of hyperkalemia?
Can newer potassium binders enable continued and optimal treatment with renin–angiotensin–aldosterone system (RAAS) inhibitors to improve important disease outcomes?
Acknowledgement
The authors thank Maryssa Canuel PhD MBA of liV Medical Education Agency for editorial support.
Footnotes
Competing interests: Jordan Weinstein reports consultancy and advisory work for AstraZeneca, Bayer, BI/Lily, Alexion, Merck, Amgen, Janssen, Otsuka and CPD Network. Louis-Philippe Girard reports consultancy and advisory work for Otsuka and Astra Zeneca. Robert McKelvie reports consultancy and advisory work for Otsuka, AstraZeneca, Bayer, Merck and Novartis. Karthik Tennankore reports consultancy and advisory work for Otsuka, Janssen, Astra Zeneca, Baxter and Bayer. Sodium zirconium cyclosilicate is licensed by AstraZeneca Canada. Patiromer is imported and distributed by Otsuka Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This review was sponsored by an unrestricted education grant from Otsuka Canada.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/