Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation technique used to treat mental health disorders, including smoking addiction.
The first rTMS device for short-term treatment for smoking cessation was approved by Health Canada in 2022.
The procedure is safe and generally well tolerated and is an emerging alternative treatment option for people who would like to stop smoking.
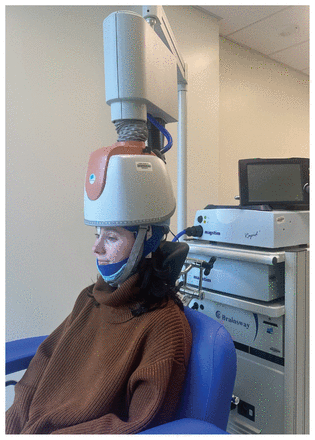
In Canada, 10.3% of the population smokes tobacco.1 Each year, 8 million deaths worldwide are attributed to smoking.2 Currently, 3 pharmacotherapies are approved for smoking cessation in Canada: varenicline, bupropion, and nicotine replacement therapy (NRT). Although these treatments are effective, many people do not respond to them, and relapse remains a problem. Recently, a repetitive transcranial magnetic stimulation (rTMS) device with a novel coil design (H-coil) was approved for smoking cessation by the US Food and Drug Administration (2020) and Health Canada (2022) (Figure 1). This is the first rTMS device approved for smoking cessation, but rTMS in various forms has been used for decades as an evidence-based treatment for depression.3
The deep repetitive transcranial magnetic stimulation device with an H-coil currently approved by Health Canada for treatment for smoking cessation, depression, and obsessive–compulsive disorder. Patients sit in a chair with the helmet secured to their head. The coil is embedded in the helmet, which is then attached to a stimulator and cooling device.
What is rTMS?
Repetitive transcranial magnetic stimulation is a noninvasive brain stimulation technique that generates magnetic fields using a magnetic coil to produce electrical currents in a targeted brain area. These currents allow for the facilitation (excitation) or suppression (inhibition) of neurons located beneath the coil, which results in changes in activity in the brain area.4 The technique can be delivered with different types of coils; the most studied is the figure of 8. This coil is effective in stimulating superficial areas of the cortex, such as the dorsolateral prefrontal cortex. Alternative coils, such as H-coils, have been designed to stimulate deeper cortical regions,5 which allow for targeting brain regions implicated in addiction, such as the insula, that are below the superficial cortex and not reached by figure-of-8 coils.6
How is treatment delivered?
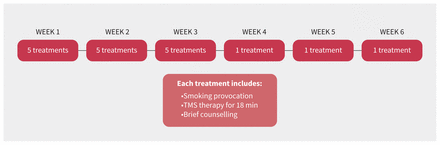
Treatment is delivered at a clinic by trained staff. The treatment protocol approved by Health Canada consists of 15 daily sessions over the course of 3 weeks, followed by 1 weekly session for 3 weeks (Figure 2). At the beginning of the first treatment, a physician will find the patient’s resting motor threshold (RMT). The RMT determines the minimum intensity required to activate a muscle (right abductor pollicis brevis) in that patient’s hand when stimulating the motor cortex. The target stimulation intensity for all treatment sessions is set at 120% of the RMT. Before each session, patients have a brief smoking provocation to activate brain circuits relevant to addiction. The patient is asked to hold a cigarette and a lighter, while imagining they are in a situation in which they would normally be smoking. Each session is about 25–30 minutes total, which includes the provocation, 18 minutes of transcranial magnetic stimulation, and brief counselling.
Treatment protocol for repetitive transcranial magnetic stimulation (rTMS) for smoking cessation.
What is the evidence to support use of rTMS for smoking cessation?
Most research evaluating rTMS as a smoking cessation therapy has looked at the effect of targeting the dorsolateral prefrontal cortex using figure-of-8 coils. These smaller studies reported mixed, inconclusive results.7 Since 2014, the H4-coil — an H-coil that targets the insula and prefrontal cortex bilaterally — has been used in 2 clinical trials. As both trials that used the H4-coil had positive results, it is the only coil that has received approval for smoking cessation from Health Canada. The first trial included 115 patients for whom pharmacotherapy was unsuccessful, using a triple-blind, randomized controlled design. High-frequency stimulation (10 Hz) with exposure to smoking cues resulted in significantly higher abstinence rates at end of treatment (43.75%) compared with sham stimulation (13.3%) (p = 0.039). It also led to a significant decrease in cigarette consumption and nicotine dependence at the end of treatment. No difference was found between low frequency and sham stimulation (p = 0.94). At 6 months after treatment, abstinence decreased to 33% in the high-frequency group and was no longer significant (p = 0.06).8
A subsequent multicentre trial included 262 people who smoked. Although having had a trial of pharmacotherapy for smoking cessation was not an inclusion criterion, all participants had tried pharmacotherapy without success. High-frequency stimulation (10 Hz) with exposure to smoking cues resulted in a 25.3% (intention to treat [ITT] 17.6%) 4-week abstinence rate compared with a 6.4% (ITT 4.8%) rate in the sham group (completed treatments p = 0.0006; ITT p = 0.002) at end of treatment. People who were abstinent at end of treatment were followed for 18 weeks after the end of treatment, and higher longer-term quit rates were found in the active group than in the sham group (completed treatments 28% v. 11.7%, p = 0.007; ITT 19.4% v. 8.7%; p = 0.017).9
In comparison, first-line interventions such as varenicline, bupropion, and NRT have abstinence rates at 6 months of 21.8%, 16.2%, and 15.7%, respectively.10 Second-line interventions for smoking cessation, such as combination therapies of varenicline and NRT, show 32.4% abstinence rates at 6 months.11 These combination therapies require 12 weeks of treatment, while deep transcranial magnetic stimulation (TMS) requires 6 weeks of treatment. Nonetheless, pharmacotherapy options have been studied extensively, have minimal adverse effects, are widely accessible across Canada, and can be taken at home, whereas deep TMS requires multiple inperson visits at a clinic that provides this specialized treatment.
Who is eligible?
People who smoke more than 10 cigarettes a day for more than a year are eligible for rTMS. The procedure is not suitable for people who smoke and have metal implants in or around their head or implanted electronic devices, such as an automated implantable defibrillator. Deep rTMS has not been tested for safety in people who smoke and who are younger than 22 years or older than 68 years, or have substance use disorders or psychotic disorders, neurologic or seizure disorders, a medical condition that puts them at increased risk of seizures, implanted electronic devices, or who are pregnant or breastfeeding. Although these are the general guidelines of eligibility, outside of research settings a prescribing physician with training in rTMS determines whether to recommend this treatment based on an individualized assessment of risk and benefits.12
What are the harms?
Repetitive transcranial magnetic stimulation has a generally favourable adverse effect profile. The most frequent adverse effects are headache and pain at the application site, face, and neck. These symptoms typically resolve once the treatment session is over.12 The most serious adverse event is seizure. According to a survey of members of the Clinical TMS Society (CTMSS) conducted in 2018,13 seizure occurred at a rate of 0.31 per 10 000 rTMS sessions and 0.71 per 1000 patients across devices. The rate of seizure among patients treated with the H-coil device for other indications was significantly higher at 5.56 per 1000 patients. This higher seizure rate is likely due to the broader induced electrical field and the higher frequency of stimulation of H-coils compared to other coils. Seizures are more likely to have been reported in people taking high doses of or abruptly stopping psychotropic medications (e.g., clonazepam, amitriptyline), which are known to increase the risk of a seizure. Seizures are also more likely to occur in people who consume high levels of alcohol within 24 hours of treatment, who have an abrupt and significant change in baseline levels of alcohol use, or are in alcohol withdrawal. To prevent seizures, the RMT should be rechecked after medication changes.13,14 In trials where rTMS was used for smoking cessation, none of the participants had a seizure.8,9,15
What resources are needed to deliver rTMS?
Treatment is delivered in a clinic that has providers with the requisite training and expertise to deliver rTMS. Treatment can be delivered by nonphysicians. However, physician oversight is needed to determine dosing of treatment, the RMT, and manage some adverse effects, such as seizure.12 Patients may be covered through extended health care plans or may need to pay for treatments out of pocket at private clinics. Given that rTMS for smoking cessation was recently approved for use in Canada, only 6 clinics currently offer this treatment, including 3 in British Columbia (Vancouver, Kelowna, and Surrey), 1 in Alberta (Edmonton), 1 in Quebec (Montréal), and 1 in Ontario (Ottawa).
What can be expected in the future?
Given the small number of clinics offering rTMS for smoking cessation, and evidence limited to clinical trials in research settings, the next steps are to analyze real-world patient outcomes of rTMS for smoking cessation and cost-effectiveness of the treatment. Given the cost and in-person visit requirement for treatment, rTMS may not be suitable as a first-line therapy but rather for those for whom medications have not resulted in smoking cessation. In addition, this treatment has not been investigated for all populations, such as people with schizophrenia who have high rates of chronic tobacco use. A proof-of-concept study has shown its potential for use in patients with schizophrenia,16 but large trials designed to determine smoking cessation outcomes have not been completed.
Additional research should investigate whether treatment protocols that combine rTMS and smoking cessation pharmacotherapies may lead to increased abstinence rates or longer periods of abstinence. Our team recently completed a trial using a different H-coil (targeting the insula) in combination with varenicline and found a significant difference in smoking abstinence rates between groups (82.4% active v. 30.7% sham stimulation at 3 months, p = 0.03). Similarly to the trials discussed above, this difference was no longer significant at the 6-month follow-up.15 These results further show the need for studies to evaluate treatment protocols targeting long-term abstinence.
CMAJ invites contributions to Innovations, which highlights recent diagnostic and therapeutic advances. Novel uses of older treatments will also be considered. For publication, the benefits of the innovation, its availability and its limitations must be highlighted clearly, but briefly. Visual elements (images) are essential. Submit brief evidence-based articles (maximum 1000 words and 5 references) to http://mc.manuscriptcentral.com/cmaj or email andreas.laupacis{at}cmaj.ca to discuss ideas.
Footnotes
Competing interests: Bernard Le Foll reports obtaining funding from Pfizer Inc. (GRAND Awards, including salary support) for investigator-initiated projects. Dr. Le Foll has obtained funding from Indivior for a clinical trial sponsored by Indivior. He has received in-kind donations of cannabis products from Aurora Cannabis Enterprises Inc. and study medication donations from Pfizer Inc. ( varenicline for smoking cessation) and Bioprojet Pharma. He was also provided with a coil for a transcranial magnetic stimulation (TMS) study from BrainsWay. He has obtained industry funding from Canopy Growth Corporation (through research grants handled by the Centre for Addiction and Mental Health [CAMH] and the University of Toronto), Bioprojet Pharma, Alcohol Countermeasure Systems, Alkermes, and Universal Ibogaine. Lastly, he has received in-kind donations of nabiximols from GW Pharmaceuticals for past studies funded by the Canadian Institutes of Health Research (CIHR) and the United States National Institutes of Health (NIH). He has participated in a session of a National Advisory Board Meeting (on Emerging Trends in Buprenorphine Extended Release) for Indivior Canada and is part of a Steering Board for a clinical trial for Indivior. He has provided consultation services for Shionogi. He is supported by CAMH, the Waypoint Centre for Mental Health Care, a clinician-scientist award from the Department of Family and Community Medicine of the University of Toronto and a Chair in Addiction Psychiatry from the Department of Psychiatry of University of Toronto. Daniel Blumberger reports receiving research support from CIHR, NIH, the Brain Canada Foundation, and the Temerty family through the CAMH Foundation and the Campbell Family Research Institute. Dr. Blumberger reports receiving in-kind equipment support for an investigator-initiated study from BrainsWay Ltd. He was the site principal investigator for 3 sponsor-initiated studies for BrainsWay Ltd. He has also received in-kind equipment support from MagVenture for 2 investigator-initiated studies. He has received medication supplies for an investigator-initiated trial from Indivior. Dr. Blumberger has also served as the co-chair of the Clinical TMS Society Clinical Standards Committee (unpaid) and chair of the Neurostimulation Procedures Advisory Committee for Mental Health and Addictions Centre of Excellent in Ontario (unpaid). Victor Tang receives research funding support through the Brain & Behavior Research Foundation, the Physician Services Incorporated Foundation, and the Labatt Family Network for Research on the Biology of Depression (all payments to institution), as well as from the Research in Addiction Medicine Scholars Program (R25DA033211) from the National Institute on Drug Abuse (payments to Dr. Tang). No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Christine Ibrahim wrote the first draft of the manuscript. Daniel Blumberger, Victor Tang, and Bernard Le Foll revised it critically for important intellectual content. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/