Prasugrel and ticagrelor are P2Y12 inhibitors that are more effective than clopidogrel at preventing ischemic events in patients presenting with acute coronary syndrome, although they cause more bleeding.
Dual antiplatelet therapy (acetylsalicylic acid and a P2Y12 inhibitor) is indicated for patients with acute coronary syndrome, usually for at least 1 year.
The type and duration of dual antiplatelet therapy is determined by a patient’s risk of recurrent ischemia and major bleeding.
Most patients with atrial fibrillation and acute coronary syndrome should be treated with a direct oral anticoagulant and clopidogrel for 12 months; acetylsalicylic acid should be added for up to 1 month after percutaneous coronary intervention.
After an acute coronary syndrome, as many as 1 in 5 patients will have a second ischemic event within 5 years.1,2 Residual risk is related to several factors that may be mitigated by pharmacologic and nonpharmacologic interventions.2 Antiplatelet therapy is a cornerstone in the management of acute coronary syndrome.1,3 Acetylsalicylic acid (ASA) — a cyclooxygenase-1 inhibitor — was introduced as an effective treatment for myocardial infarction almost 5 decades ago and remains the most widely used antiplatelet therapy.4,5 Although ASA is effective in reducing mortality rates,6 combining ASA with a second antiplatelet agent, a P2Y12 receptor inhibitor (known as dual antiplatelet therapy [DAPT]) provides additional benefit and is now the preferred initial strategy for acute coronary syndromes over ASA alone.7
We review emerging evidence regarding the use of antiplatelet therapy in acute coronary syndromes, as well as updates to the Canadian and European Society of Cardiology guidelines that highlight adjustments in the choice and duration of antiplatelet therapy, in addition to ASA (Box 1). We particularly focus on strategies to reduce bleeding risk after percutaneous coronary intervention (PCI).3,8
Literature search
We conducted a targeted, nonsystematic MEDLINE search from its inception until August 2021 using the terms “antiplatelet,” “clopidogrel,” “prasugrel,” “ticagrelor,” “acute coronary syndrome,” “myocardial infarction” or “bleeding.” We limited the search to articles in English and focused on randomized clinical trials or systematic reviews, although we did not apply any restriction on study type.
What are the options for oral antiplatelet therapy?
Clopidogrel is a second-generation thienopyridine that has a better safety profile than ticlopidine, a first-generation thienopyridine.9 Clopidogrel reduces ischemic events by almost 20% when added to ASA for patients presenting with acute coronary syndromes, with or without ST-segment elevation.7,10,11 It irreversibly antagonizes the receptor for platelet adenosine diphosphate (ADP)-P2Y12. The use of clopidogrel may be associated with gastrointestinal symptoms and skin rashes.
Prasugrel and ticagrelor are more potent P2Y12 inhibitors than clopidogrel. Prasugrel is a third-generation thienopyridine that exerts its antiplatelet properties by irreversibly antagonizing the ADP-P2Y12 receptor; similar to clopidogrel, it requires hepatic conversion to its active metabolites. Ticagrelor is part of the cyclopentyltriazolopyrimidine family and does not require hepatic conversion to its active metabolites before reversibly inhibiting the ADP-P2Y12 receptor. Ticagrelor may cause shortness of breath or increased levels of uric acid, which leads to gout.
Both prasugrel and ticagrelor provide faster and more consistent inhibition of platelet aggregation and are associated with a further 15%–20% relative risk reduction of ischemic events compared with clopidogrel.12,13 Ticagrelor also reduces cardiovascular and all-cause mortality rates.12 Although ticagrelor and prasugrel are associated with greater bleeding risk than clopidogrel, both are recommended over clopidogrel in patients with low bleeding risk. Recently, the ISAR-REACT-5 (Intracoronary Stenting and Antithrombotic Regimen 5) study reported fewer ischemic events associated with prasugrel, with no difference in the incidence of major bleeding, when compared with ticagrelor.14 However, a number of uncertainties preclude definitively recommending one drug over the other.15 Prasugrel is not recommended in patients older than 75 years of age and in those with a body weight less than 60 kg because of an increased risk of fatal and intracranial bleeding.13 A recent meta-analysis summarized the relative differences in ischemic and bleeding risks among the 3 different P2Y12 inhibitors (Table 1).16
Relative difference in ischemic and bleeding risks* among different P2Y12 inhibitors16
Clopidogrel is currently recommended for patients with acute coronary syndromes who are at high bleeding risk or those who cannot take a potent P2Y12 inhibitor because of adverse effects or cost.3,8 Guidelines recommend treatment with clopidogrel as the initial antiplatelet drug for patients with ST-segment elevation myocardial infarction who are treated with fibrinolytic therapy. However, results of the TREAT (Ticagrelor in Patients With ST-Elevation Myocardial Infarction Treated With Pharmacological Thrombolysis) study indicated that switching from clopidogrel to ticagrelor within 24 hours did not lead to an increase in major bleeding in the first 30 days postlysis, compared with continuing clopidogrel.17
Current guidelines recommend the use of DAPT after an acute coronary syndrome, irrespective of the revascularization strategy, including for medically managed patients and those who undergo coronary artery bypass grafts.3,18 A subgroup analysis of 7985 patients from the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial who did not undergo revascularization after randomization showed that adding clopidogrel to ASA reduced ischemic events by an absolute 1.9% after 12 months of follow-up, compared with placebo and ASA.7 Ticagrelor provides consistent benefit over clopidogrel in reducing ischemic events, irrespective of revascularization strategy.12,19
For patients with non-ST segment elevation acute coronary syndrome, (NSTACS) the timing of coronary angiography is a factor in the choice of antiplatelet treatment. For patients scheduled for coronary angiography within 24 hours of presentation, there is debate regarding whether patients should be preloaded with a potent P2Y12 inhibitor. Preloading with prasugrel or ticagrelor did not reduce ischemic events in patients with NSTACS scheduled for early coronary angiography.20,21 Thus, the European guidelines discourage routine preloading of P2Y12 inhibitors in patients with NSTACS who are planned for an early coronary angiogram and suggest starting DAPT once the need for angioplasty or stents is confirmed.3 This does not apply for patients presenting with ST-segment elevation myocardial infarction in whom preloading with DAPT is recommended. Preloading should also be considered in non-PCI centres because coronary angiography may be delayed. Further, given that, in Canada, most patients with NSTACS do not routinely undergo angiography within the first 24 hours of presentation, preloading with DAPT in patients with moderate-to-high risk of ischemia is reasonable.8 If there is suspicion of left main disease or possible aortic dissection, DAPT should not be given.3
How long should dual antiplatelet therapy be continued?
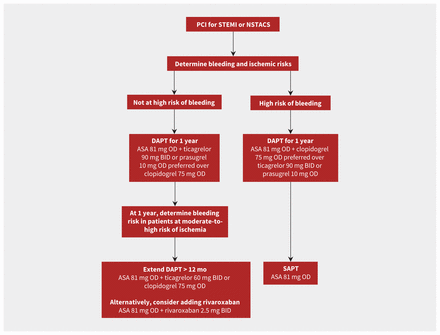
Current guidelines recommend DAPT for 1 year after an acute coronary syndrome, particularly in patients with heightened ischemic risk (Box 2 and Figure 1).3,8 However, patients at high risk of bleeding may be considered for shorter treatment duration. Two large randomized controlled trials evaluated extending the duration of DAPT beyond 12 months, using ticagrelor in patients with a history of myocardial infarction in the PEGASUS-TIMI 54 (Prevention with Ticagrelor of Secondary Thrombotic Events in High-Risk Patients with Prior Acute Coronary Syndrome – Thrombolysis In Myocardial Infarction 54) trial, and using clopidogrel or prasugrel in stable patients and patients with acute coronary syndromes who received a PCI in the Dual Antiplatelet Therapy trial.22,23 Both trials reported significant relative reductions in ischemic events of 15%–30%, but these benefits were offset by a significant increase in major bleeding.22,23 A recent meta-analysis confirmed these findings; DAPT extension beyond 12 months was associated with a 32% reduction in myocardial infarction and a 63% increase in major bleeding among patients who received a PCI.24
Features of patients at high risk of ischemic events
Previous stent thrombosis on adequate antiplatelet therapy
Stenting of the last remaining patent coronary artery
Diffuse multivessel disease, especially in patients with diabetes
Chronic kidney disease (i.e., creatinine clearance < 60 mL/min)
At least 3 stents implanted
At least 3 lesions treated
Bifurcation with 2 stents implanted
Total stented length greater than 60 mm
Treatment of a chronic total occlusion
History of ST-segment elevation myocardial infarction
Antiplatelet recommendations in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). Note: ASA = acetylsalicylic acid, BID = twice daily, DAPT = dual antiplatelet therapy, NSTACS = non-ST segment elevation acute coronary syndrome, OD = once daily, SAPT = single antiplatelet therapy, STEMI = ST-segment elevation myocardial infarction.
Improvements in stent design and increased recognition of the importance of preventing bleeding led researchers to evaluate shorter durations of DAPT; the findings of noninferiority studies of stable patients and patients with acute coronary syndrome after PCI have supported this approach.25 Meta-analyses found comparable incidences of stent thrombosis and adverse ischemic events; however, myocardial infarction was more frequent in the shortened DAPT group.25 Recently, the MASTER-DAPT (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Prolonged DAPT Regimen) study randomized 4434 patients at high risk of bleeding who were free of cardiovascular events at 1 month after PCI to receive 1 month of DAPT or standard DAPT of at least 3 months after PCI.26 The measure of adverse clinical events (defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding) was comparable between the 2 groups.26 Importantly, the incidence of major bleeding or clinically relevant, nonmajor bleeding was significantly lower in the shorter treatment group (6.5% v. 9.4%, p < 0.001).26
The use of a single antiplatelet agent was recently tested in an open-label, multicentre randomized study of 5438 patients who had completed 6–18 months of DAPT without any clinical events, compared with ASA alone. Over a mean follow-up of 24 months, clopidogrel reduced the composite outcome of all-cause death, myocardial infarction, stroke, readmission because of an acute coronary syndrome and major bleeding events (5.7% v. 7.7%; hazard ratio [HR] 0.73, 95% confidence interval [CI] 0.59–0.90).27 Both ischemic (3.7% v. 5.5%; HR 0.68, 95% CI 0.52–0.87) and any bleeding events (2.3% v. 3.3%; HR 0.70, 95% CI 0.51–0.98) were reduced with the use of clopidogrel.
Other strategies for extended secondary prevention include the use of a low dose of oral anticoagulant in combination with ASA. This has been used in the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial for patients with stable atherosclerotic cardiovascular disease, including previous myocardial infarction, cerebrovascular and peripheral artery disease. 28 Compared with ASA alone, rivaroxaban (2.5 mg twice daily) and ASA reduced ischemic events by an absolute 1.3%, but was associated with more major bleeding (3.1% v. 1.9%).28
How is bleeding risk assessed and managed?
Models have been developed to quantify bleeding risk;1,29 they include clinical and biomarker variables and have moderate-to-good accuracy (Table 2). The DAPT, PRECISE-DAPT and PRAISE models are accessible online. In a meta-analysis of 88 563 patients, the DAPT score consistently identified patients at high risk of bleeding and ischemia in different cohorts of patients.39 Similarly, the PRECISE-DAPT model effectively identified patients who were not suitable for extended DAPT and were likely to be at risk of bleeding without a decrease in ischemic events.35 The PRECISE-DAPT model was also validated in cohorts of patients with acute coronary syndromes who underwent PCI and were treated with potent P2Y12 inhibitors, and showed moderate accuracy in predicting future bleeding risk.40
Models to estimate bleeding risk
The PRAISE model used machine learning to predict bleeding, ischemic risk and all-cause deaths.38 The model was derived from 2 cohorts of patients with acute coronary syndromes who were treated with clopidogrel or more potent P2Y12 inhibitors. It accurately predicted major bleeding, as well as acute MI and all-cause mortality. Importantly, machine learning risk-scoring models need to be tested in randomized controlled trials to assess their impact on clinical outcomes.
Strategies to reduce bleeding risk at the time of PCI include the use of radial access (preferable to accessing a more central artery), using fluoroscopy or ultrasonography guidance to access the common femoral artery41 and selecting the right stent platform that is indicated for patients with high bleeding risk or is considered safe for early discontinuation of DAPT.26,42 Bleeding risk can also be reduced with the use of clopidogrel rather than prasugrel or ticagrelor in patients who are at high risk.41 After hospital discharge, several strategies should be used to reduce bleeding risk (Box 3).
Strategies to reduce bleeding risk following hospital discharge
Shorter duration of dual antiplatelet therapy
Use of clopidogrel rather than ticagrelor or prasugrel
Avoidance of nonsteroidal anti-inflammatory drugs
Optimal blood pressure management
Abstinence from alcohol
Use of mobility aids, when appropriate
Use of proton pump inhibitor for all patients after percutaneous coronary intervention, as recommended by current guidelines.18
Screening and eradication of Helicobacter pylori (a large randomized controlled trial is underway to assess this strategy in patients with acute myocardial infarction)43
Correction of anemia to reduce the impact of bleeding
The management of acute bleeding events is discussed elsewhere. 44 Current guidelines categorize patients according to the type of bleeding event and recommend management according to severity (Table 3).18 Reversal agents are currently available for some oral anticoagulants, and antidotes to P2Y12 inhibitors are being developed.45 A ticagrelor reversal agent, bentracimab (PB2452), is a human monoclonal antibody that provides immediate and sustained reversal of the antiplatelet effects of ticagrelor in healthy volunteers.45 It is currently being studied in the REVERSE-IT (Rapid and Sustained Reversal of Ticagrelor–Intervention Trial) trial (NCT04286438). Management of bleeding events in patients on DAPT can be challenging and involvement of specialists should be considered (Box 4).
Management of bleeding events in patients with acute coronary syndrome receiving antithrombotic therapy
Indications for referral to cardiologist and other specialist regarding antiplatelet treatment
Bleeding events in patients within 1 year of acute coronary syndrome; advice from a cardiologist about resumption or discontinuation of a second antiplatelet agent should be sought early, and consultation with a gastroenterologist should also be considered, if indicated
New onset atrial fibrillation
Patients with planned noncardiac surgery (particularly major surgeries)
Patients with chronic bleeding diatheses, such as hemophilia or severe liver disease; consultation with a hematologist should be considered
Patients who develop thrombocytopenia; consultation with a hematologist should be considered
Patients who develop a cerebrovascular event; consultation with a neurologist should be considered
Can treatment with acetylsalicylic acid be stopped early?
Early stopping of ASA while maintaining P2Y12 inhibition has recently been tested, based on experimental data that suggested the synergistic effect of inhibiting cyclooxygenase-1 with ASA and P2Y12 inhibitors is less relevant in the presence of potent P2Y12 inhibitors.46 A recent meta-analysis of data from 16 898 patients with acute coronary syndromes showed that P2Y12 inhibitor monotherapy after 1–3 months of DAPT reduced bleeding events by 50% with no significant increase in ischemic events, compared with 12 months of DAPT.24,47 A recent meta-analysis included individual data from 24 096 patients enrolled in 6 randomized trials and highlighted the superiority of P2Y12 inhibitor monotherapy over DAPT in reducing ischemic events in women, and the reduction of bleeding events when a potent P2Y12 inhibitor was part of the DAPT regime.48 Nonetheless, P2Y12 inhibitor monotherapy is still not widely used in the management of patients after PCI.
What are the indications for dual antiplatelet therapy in patients with atrial fibrillation?
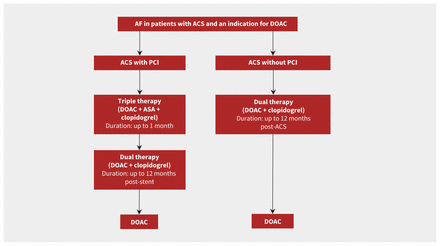
Dual antiplatelet therapy does not prevent stroke and systemic thromboembolism in patients with atrial fibrillation as effectively as oral anticoagulation.49 Conversely, an oral anticoagulant does not reduce coronary ischemic events, including stent thrombosis, as effectively as DAPT. Thus, triple therapy combining DAPT and OAC is recommended in patients with acute coronary syndromes, either medically managed or after PCI, who also have atrial fibrillation. Direct oral anticoagulants are associated with a lower rate of bleeding events than vitamin K antagonists, and are therefore preferred in patients with atrial fibrillation,50 except in those with mechanical heart valves, moderate-to-severe mitral stenosis or advanced renal disease. Clopidogrel is the P2Y12 of choice in patients receiving triple therapy, rather than ticagrelor or prasugrel, because of the lower risk of bleeding.3
The duration of triple therapy remains a matter of debate, given the increased risk of major bleeding over time. The AUGUSTUS (Open-label, 2 × 2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Percutaneous Coronary Intervention) trial showed that, beyond the first 30 days (the period of highest risk for stent thrombosis), ASA and oral anticoagulation increased bleeding events without significantly reducing ischemic events when compared with placebo and oral anticoagulation in patients receiving P2Y12 inhibitors.51,52
Numerous studies, including a recent meta-analysis, found that combining oral anticoagulation and single antiplatelet therapy reduced bleeding risk compared with triple therapy.53 Importantly, these studies were not powered to detect potential differences in ischemic events, and the meta-analysis found an increased risk of stent thrombosis associated with the combination therapy.53 A small study of consecutive patients suggested that the ischemic risk was greater in patients who underwent complex PCI procedures without the use of ASA immediately after a PCI procedure.54 Therefore, triple therapy should be considered in patients at high risk of ischemia and of stent thrombosis and low risk of bleeding for up to 1 month after PCI (Figure 2). After 1 month, ASA should be stopped, and oral anticoagulation and a P2Y12 inhibitor (preferably clopidogrel, given its lower bleeding risk) should be continued up to 12 months in patients after acute coronary syndrome. At 1 year, oral anticoagulation monotherapy should be used for secondary prevention of stroke.55
Antiplatelet management in patients with acute coronary syndrome (ACS) and atrial fibrillation (AF). Direct oral anticoagulation (DOAC) is preferred over warfarin; however, if warfarin is to be used the recommended international normalized ratio target is 2.0–2.5. The timing of when to discontinue acetylsalicyclic acid (ASA) will depend on the individual patient’s ischemic and bleeding risk. Note: PCI = percutaneous coronary intervention.
How should antiplatelet agents be switched?
De-escalation from a more potent P2Y12 inhibitor to clopidogrel occurs in up to 28% of patients with acute coronary syndromes, most often because of bleeding or high risk of bleeding.56 Similarly, switching between potent P2Y12 inhibitors may be required if specific adverse effects such as shortness of breath or gout develop in patients receiving ticagrelor. An international consensus document and Canadian guidelines provide guidance to physicians when switching between P2Y12 inhibitors (Box 5).30,57
Switching between oral P2Y12 inhibitors
Ticagrelor to clopidogrel
Ticagrelor has a relatively fast offset of action. Clopidogrel should be administered 24 hours after the last dose of ticagrelor. A 600 mg loading dose of clopidogrel should be considered unless the patient had a recent bleeding event, in which case clopidogrel 75 mg should be considered.
Prasugrel to clopidogrel
The prolonged offset of prasugrel means that the usual clopidogrel maintenance dose of 75 mg daily should be started 24 hours after the last dose of prasugrel.
Ticagrelor to prasugrel
A 60 mg loading dose of prasugrel should be administered 24 hours after the last dose of ticagrelor.
Prasugrel to ticagrelor
A 90 mg maintenance dose of ticagrelor should be administered twice daily 24 hours after the last prasugrel dose. If it has been fewer than 30 days since the patient’s PCI, a loading dose of 180 mg ticagrelor should be considered.
Guided de-escalation therapy by either platelet function testing or CYP2C19-directed genotyping may also be considered in select patients with acute coronary syndromes.58
Conclusion
Prasugrel and ticagrelor are antiplatelet agents that are more effective than clopidogrel at decreasing the future risk of ischemic events in patients with acute coronary syndromes, but are more likely to cause bleeding. The choice of antiplatelet regimen is influenced by the ischemic and bleeding risk of each patient (Figure 3). Up to a month of triple therapy with ASA, clopidogrel and an oral anticoagulant should be considered in patients with acute coronary syndromes who also have atrial fibrillation.
Flowchart for antiplatelet management in patients with acute coronary syndrome. Note: ASA = acetylsalicylic acid, CABG = coronary artery bypass grafting, OAC = oral anticoagulant, PCI = percutaneous coronary intervention. *Patients receiving fibrinolytic therapy should be loaded with ASA and clopidogrel. Switching to ticagrelor within 24 hours should be considered.
Footnotes
Competing interests: Alan Bell reports consulting fees from AstraZeneca, Bayer and Sanofi; speaker fees from AstraZeneca; and board membership with Thrombosis Canada, Hypertension Canada and the Canadian Cardiovascular Society. Sol Stern reports honoraria from Bayer, Pfizer, Bristol Myers Squibb and Sea Courses Inc. Shaun Goodman reports research grant support from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Eli Lilly, Esperion, Ferring Pharmaceuticals, Merck, Novartis, Pfizer, Regeneron, Sanofi, Heart and Stroke Foundation of Ontario, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Center for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Center, PERFUSE Research Institute and TIMI Study Group (Brigham Health). He also reports consulting honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Eli Lilly, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, PendoPharm of Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharma, Canadian Heart Research Centre and MD Primer, and speaking fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Novartis, PendoPharm of Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharm, Canadian Heart Research Centre and MD Primer. He sits on boards with American Regent of Daiichi-Sankyo and Novo Nordisk and is co-director of the Canadian VIGOUR Centre. All competing interests are outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/