Abstract
Background: Maternal obesity is associated with stillbirth, but uncertainty persists around the effects of higher obesity classes. We sought to compare the risk of stillbirth associated with maternal obesity alone versus maternal obesity and additional or undiagnosed factors contributing to high-risk pregnancy.
Methods: We conducted a retrospective cohort study using the Better Outcomes Registry and Network (BORN) for singleton hospital births in Ontario between 2012 and 2018. We used multivariable Cox proportional hazard regression and logistic regression to evaluate the relationship between prepregnancy maternal body mass index (BMI) class and stillbirth (reference was normal BMI). We treated maternal characteristics and obstetrical complications as independent covariates. We performed mediator analyses to measure the direct and indirect effects of BMI on stillbirth through major common-pathway complications. We used fully adjusted and partially adjusted models, representing the impact of maternal obesity alone and maternal obesity with other risk factors on stillbirth, respectively.
Results: We analyzed data on 681 178 births between 2012 and 2018, of which 1956 were stillbirths. Class I obesity was associated with an increased incidence of stillbirth (adjusted hazard ratio [HR] 1.55, 95% confidence interval [CI] 1.35–1.78). This association was stronger for class III obesity (adjusted HR 1.80, 95% CI 1.44–2.24), and strongest for class II obesity (adjusted HR 2.17, 95% CI 1.83–2.57). Plotting point estimates for odds ratios, stratified by gestational age, showed a marked increase in the relative odds for stillbirth beyond 37 weeks’ gestation for those with obesity with and without other risk factors, compared with those with normal BMI. The impact of potential mediators was minimal.
Interpretation: Maternal obesity alone and obesity with other risk factors are associated with an increased risk of stillbirth. This risk increases with gestational age, especially at term.
The relationship between maternal obesity and stillbirth has been well established.1–3 Compared with pregnant people with normal body mass index (BMI), those with obesity (BMI ≥ 30.0) have 2–5 times the risk of stillbirth.2,4,5 Studies have shown increased hazard ratios (HRs) for stillbirth with advancing gestational age in pregnancies affected by obesity.2 In addition to BMI, sociodemographic, lifestyle and obstetric factors, and pre-existing health conditions have also been identified as important risk factors for stillbirth.5,6
Previous studies have evaluated several risk factors for stillbirth and have explored the impact of obesity on stillbirth risk by gestational age.2,5,6 Little literature, however, has explored the relationship between obesity and stillbirth by gestational age while controlling for possible confounding variables and analyzing for potential mediators. The impact of higher obesity classes on the stillbirth rate should also be assessed, as should differences in stillbirth risk for those with obesity alone versus those with obesity and additional or undiagnosed factors contributing to high risk for stillbirth. Sometimes people with obesity have pre-existing conditions not yet diagnosed in early pregnancy, such as pregestational diabetes or chronic hypertension, which contribute to stillbirth risk. Diabetes or hypertension diagnosed during pregnancy, but before 20 weeks’ gestation, constitute pre-existing conditions, rather than gestational illnesses.7,8 Pregnant people may develop complications, such as fetal growth restriction, only after the initial pregnancy visit. Certain complications, such as preeclampsia, are diagnosed after 20 weeks’ gestation and also increase stillbirth risk. Those with obesity are more likely to have these pre-existing conditions and develop pregnancy complications, compared with those with normal BMI. Determining stillbirth risk among those with obesity and other risk factors for stillbirth may be useful for early pregnancy counselling around obstetrical risks and complications, as well as discussions around optimal timing of delivery.
We aimed to evaluate the effect of BMI on risk of stillbirth by gestational age groups, analyzing for potential confounders and possible mediators. We sought to determine the risk of stillbirth among pregnant people with obesity, either adjusting or not adjusting for other risk factors, compared with pregnant people with normal BMI.
Methods
We conducted a population-based, retrospective cohort study using data from the Better Outcomes Registry and Network (BORN), a validated database that includes antenatal, intrapartum, postpartum, and neonatal outcome data on births in the province of Ontario, Canada, that occurred after 20 weeks’ gestation.9–11 We included singleton pregnancies delivered at 20 weeks’ gestation or later or a birth weight of at least 500 g in an Ontario hospital between Apr. 1, 2012, and Dec. 31, 2018. We excluded terminations of pregnancy after 20 weeks’ gestation.
The primary outcome was stillbirth, defined as fetal death at 20 weeks’ gestation or at a birth weight of at least 500 g.12 The primary exposure was prepregnancy BMI, classified into the following 6 categories: underweight (< 18.5), normal (18.5–24.9), overweight (25.0–29.9), class I obesity (30.0–34.9), class II obesity (35.0–39.9), and class III obesity (BMI ≥ 40.0), with normal BMI as the reference. We analyzed gestational age in completed gestational weeks, grouped to reserve sufficient power and maintain clinical interpretability (20–23, 24–27, 28–31, 32–35, 36, 37, 38, 39, 40, ≥ 41 wk).
Statistical analysis
We performed statistical analyses using SAS version 9.4 (SAS Institute). We presented maternal characteristics by BMI category and by stillbirth versus live birth. We described stillbirths overall and by antepartum versus intrapartum occurrence, and described the frequencies of maternal, fetal, and obstetrical complications for the overall stillbirth population and for each BMI category. Complications included hypertensive disorders of pregnancy, gestational diabetes, small-for-gestational-age (SGA) infants (< 10th and < 3rd percentiles), placental abruption, and fetal and neonatal congenital anomalies.5,6 We removed cases with missing values for any of these variables from the cohort. To assess the potential bias of the study results because of missing values, we performed a sensitivity analysis, comparing the study cohort to the cohort with missing values for the covariates, calculating standardized differences between the groups, using a value of more than 0.1 to indicate an imbalance between the groups.
We used multivariable Cox proportional hazard regression analysis to examine the relationship between BMI class and stillbirth while controlling for potential confounders. The time variable was gestational age in weeks at birth. The time to event was stillbirth and we censored live birth at the gestational age at birth. We included all pregnancies in 1 model with a single HR estimate for each group in comparison to the normal BMI group. We also used a logistic regression model to examine the gestational age–specific odds ratios (ORs) and 95% confidence intervals (CIs) for stillbirth, with a separate model fitted for each gestational age group. In the logistic regression model, stillbirth was a dichotomous outcome, stratified by the gestational age group. We compared the risk of stillbirth among the births within each specific gestational age group. We plotted point estimates by gestational age to assess the relative increases in OR with advancing gestation for each obesity class, compared with normal BMI.
We calculated fully adjusted and partially adjusted HRs and their 95% CIs for stillbirth for pregnant people with overweight, class I, class II, or class III obesity, with those with normal BMI as the reference. The fully adjusted model accounted for identified possible confounders associated with stillbirth, including maternal age greater than 35 years, nulliparity, any smoking, low median family income quintile, substance use, artificial reproductive technologies, chronic hypertension, pregestational diabetes, other pre-existing health conditions, previous stillbirth, previous cesarean delivery, antenatal care provider, SGA (< 10th percentile), gestational diabetes, hypertensive disorders of pregnancy, placental abruption, and congenital fetal anomalies. The partially adjusted model adjusted for demographic characteristics and variables reliably known or already diagnosed in early pregnancy; we did not adjust for smoking, substance use, chronic hypertension, pre-gestational diabetes, previous stillbirth, SGA, gestational diabetes, hypertensive disorders of pregnancy, placental abruption, and congenital fetal anomalies, because they may be diagnosed only later in pregnancy. We also applied full and partial adjustments to the logistic regression model.
We conducted mediation analyses based on the counterfactual framework to estimate natural direct and indirect associations for major potential mediators,13 including SGA below the 10th and 3rd percentiles, preterm birth, and hypertensive disorders of pregnancy. We show the conceptual causal pathway, demonstrating the interplay of mediating and confounding effects toward the outcome, in Appendix 1, Supplemental Figure 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221450/tab-related-content.
Ethics approval
This study was approved by the Ottawa Health Science Network Research Ethics Board (no. 20180126–01H).
Results
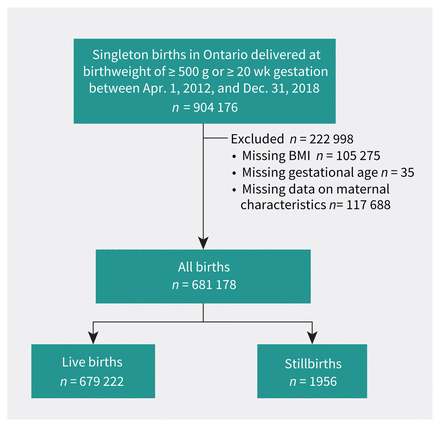
We included 681 178 eligible births in the final analysis; there were 1956 (0.29%) stillbirths (Figure 1). Those with obesity had increased parity, lower median family income, and higher rates of smoking than those with normal BMI (Table 1). Stillbirths occurred more commonly in pregnant people with obesity, nulliparity, smoking, lower median family income, substance use, use of artificial reproductive technologies, chronic hypertension, pre-gestational diabetes, other pre-existing medical conditions, and a history of previous stillbirth (Table 2).
Study flowchart. Intentional terminations were not included in the cohort. BMI = body mass index.
Demographic characteristics by body mass index (BMI) category among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018
Demographic characteristics by live birth or stillbirth among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018
About 12% of BMI data and 15% of covariate data were unavailable (Figure 1). The sensitivity analysis showed an imbalance between the study cohort and the cohort with missing values for third income quintile and non-exposure to substance use in pregnancy, with standardized differences of 0.11 and 0.24, respectively (Appendix 1, Supplemental Table 1A). More data were missing for the earlier versus later years of the study (Appendix 1, Supplemental Table 1B).
We characterized stillbirths as occurring either antepartum or intrapartum, and by BMI category (Table 3). We observed that individuals with class I and II obesity were more likely to have an antepartum than an intrapartum stillbirth, compared with those with normal BMI. We described complication rates within the stillbirth population overall, as well as by BMI category (Table 4),14–18 and observed higher rates of growth-restricted fetuses and congenital anomalies in the stillbirth cohort than in the general pregnant population in Canada. Stillbirths among pregnant people in the higher obesity classes were associated with a greater proportion of hypertensive disorders of pregnancy and gestational diabetes, compared with stillbirths among those with normal BMI.
Distributions of antepartum versus intrapartum stillbirths by body mass index (BMI) category among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018
Obstetrical complications associated with stillbirth by body mass index (BMI) category among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018
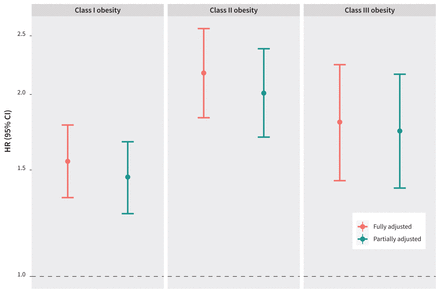
We show the fully adjusted and partially adjusted hazard ratios (HRs) with 95% CIs for stillbirth by obesity class in Figure 2 (also shown numerically in Appendix 1, Supplemental Table 3). Obesity was strongly associated with increased risk of stillbirth in both models. Figure 2 shows that the HRs and CIs between the fully adjusted and partially adjusted models were similar within each obesity class. The highest risk of stillbirth was observed among pregnant people with class II obesity.
Fully adjusted and partially adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for stillbirth among pregnant people in maternal obesity classes I, II, and III, compared with those with normal body mass index, among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018. The fully adjusted Cox regression model is adjusted for maternal age older than 35 years, nulliparity, smoking, low income quintile, substance use, use of artificial reproductive technologies, chronic hypertension, pre-gestational diabetes, other pre-existing health conditions, previous stillbirth, previous cesarean delivery, antenatal care, small for gestational age (< 10th percentile), gestational diabetes, hypertensive disorders of pregnancy, placental abruption, and congenital fetal anomalies. The partially adjusted Cox regression model is adjusted for maternal age older than 35 years, nulliparity, low income quintile, use of artificial reproductive technologies, other pre-existing health conditions (not including chronic hypertension and pre-gestational diabetes), previous cesarean delivery, and antenatal care (representing the population with additional or undiagnosed high-risk pregnancy factors).
Figure 3 shows the ORs and 95% CIs from the multivariable logistic regression analysis for stillbirth for each obesity class for 10 gestational periods between 20 weeks’ and 41 or more weeks’ gestation. Among people with class I obesity, increased ORs for stillbirth became statistically significant at 39 weeks’ gestation, double the risk of stillbirth compared with those with normal BMI. For obesity classes II and III, stillbirth risk at 36 weeks’ gestation was 2–2.5 times that of people with normal BMI. Among people with class II obesity, the highest ORs for stillbirth occurred at 38 weeks’ gestation (3–3.5 times higher than those with normal BMI) and at 40 weeks’ gestation (4–4.5 times higher than those with normal BMI). The highest ORs for stillbirth among those with class III obesity were seen around the same gestational ages as the class II group, and were further increased at 41 or more weeks’ gestation.
Fully adjusted and partially adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for stillbirth by gestational age among pregnant people in maternal obesity classes I, II, and III, compared with normal body mass index, among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018. Note the change in gestational age interval between 32–35 and 36 or more weeks. A separate model was fitted for each gestational age group. The fully adjusted logistic regression model is adjusted for maternal age older than 35 years, nulliparity, smoking, low income quintile, substance use, use of artificial reproductive technologies, chronic hypertension, pre-gestational diabetes, other pre-existing health conditions, previous stillbirth, previous cesarean delivery, antenatal care, small for gestational age (< 10th percentile), gestational diabetes, hypertensive disorders of pregnancy, placental abruption, and congenital fetal anomalies. The partially adjusted logistic regression model is adjusted for maternal age older than 35 years, nulliparity, low income quintile, use of artificial reproductive technologies, other pre-existing health conditions (not including chronic hypertension and pre-gestational diabetes), previous cesarean delivery, and antenatal care (representing the population with additional or undiagnosed high-risk pregnancy factors).
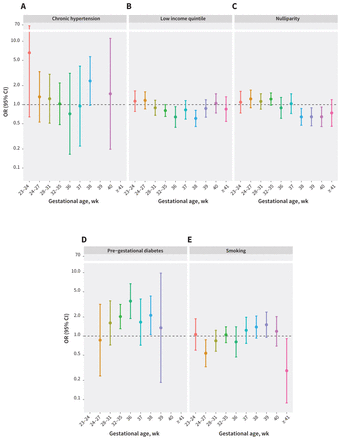
Figure 4 shows the OR of stillbirth over the 10 gestational periods for other major risk factors for stillbirth. For chronic hypertension and pre-gestational diabetes, risk of stillbirth peaked at 38 weeks’ gestation with ORs of 2.4 (95% CI 1.0–5.7) and 2.1 (95% CI 1.1–4.3), respectively. For comparison, at 38 weeks’ gestation, people with class II and III obesity had higher risk of stillbirth, with fully adjusted ORs of 3.5 (95% CI 2.2–5.6) and 2.6 (1.4–4.8), respectively (Figure 3). Among people with chronic hypertension or pre-gestational diabetes, we observed a drop in the OR for stillbirth after 38 weeks’ gestation (Figure 4). For class II and III obesity, however, we observed an even higher OR for stillbirth at 40 weeks’ gestation than at 38 weeks’ (Figure 3).
Odds ratios (ORs) and 95% confidence intervals (CIs) for stillbirth by gestational age for select major pre-existing maternal risk factors among singleton hospital births in Ontario, Canada, Apr. 1, 2012, to Dec. 31, 2018. Note the change in gestational age interval between 32–35 and 36 or more weeks. This is based on the logistic regression model. A separate model was fitted for each gestational age group.
Mediation analyses showed that, compared with normal BMI, preterm births and hypertensive disorders mediated 21% and 5% of the partially adjusted associations between obesity and stillbirth, respectively, and 12% and 1% of the fully adjusted associations. Having an SGA infant had a negative effect in mediator analysis, with SGA below the 10th percentile found to have a statistically significant indirect effect. We present the breakdown of direct, indirect and total effects for each of these mediators in Appendix 1, Supplemental Table 2.
Interpretation
Pregnant people with obesity, particularly those in the highest obesity classes, have greater overall risk of stillbirth than those with normal BMI, especially with advancing gestational age, after controlling for possible confounders, either fully or partially adjusting to reflect that some risk factors may be undiagnosed at the first pregnancy visit. We found small mediating effects of preterm birth and hypertensive disorders on the common pathway to stillbirth, not reaching a level that implied clear, definitive influence. Conversely, having an SGA infant had a negative effect in the mediator analysis, possibly related to increased clinical surveillance of SGA fetuses, which successfully mitigated the increased risk of stillbirth associated with this complication.
Given the persistently increased risk of stillbirth with obesity alone, as well as with obesity with risk factors, those with additional risk factors may benefit from timely referral and greater surveillance closer to term; additional risk factors may also warrant earlier delivery. Chronic hypertension and pre-gestational diabetes had peak stillbirth risks at 38 weeks’ gestation. This compares with a stillbirth risk ratio (which approximates OR for rare outcomes such as stillbirth) of about 1.3 in the general pregnant population at the same gestational age.19 The subsequent drop in risk after 38 weeks for chronic hypertension and pre-gestational diabetes is likely related to clinical practice recommendations to deliver pregnant people with these conditions around 38–39 weeks’ gestation.20,21 The increase in risk of stillbirth with class II and III obesity at 38 weeks’ gestation was higher than the increase in risk associated with chronic hypertension and pre-gestational diabetes. The increase in risk of stillbirth among those with class II and III obesity was higher still at 40 weeks’ gestation.
Given that current national guideline recommendations around timing of delivery among those with chronic hypertension and pre-gestational diabetes are based on a level of risk tolerance for stillbirth in these populations that is lower than the level of risk seen among people with class II and III obesity at the same gestational age, delivery before 40 weeks’ gestation may be warranted among those with obesity. Although we noted lower ORs for stillbirth among those with class III obesity at 37 and 39 weeks’ gestation, these were likely owing to smaller sample sizes, reflected in the wider 95% CIs. The tighter 95% CIs noted among those with class I obesity (Figure 3) were likely owing to the larger sample size. Our findings suggest that delivery around 39 weeks’ gestation for pregnant people with class I obesity and 38 weeks’ gestation for pregnant people with class II or III obesity may help mitigate the risk of stillbirth.
Additional analysis of neonatal outcomes for delivery at these gestational ages may solidify such recommendations. A retrospective population-based study using BORN data, evaluating timing of delivery for pregnant people with isolated chronic hypertension, showed delivery at 38–39 weeks’ gestation reduced the risk of superimposed preeclampsia and associated maternal and perinatal complications, including stillbirth, without increasing the rate of cesarean delivery.22 Given the many other indications for earlier term delivery, it would not be unreasonable to plan for early-term delivery among pregnant people with class II and III obesity, particularly those with additional risk factors.
Studies have shown that obesity is an independent risk factor for stillbirth.3–6 A 2014 decision analysis suggested that 38 weeks’ gestation may be the optimal gestational age for delivery among pregnant people with obesity, such that 203 stillbirths would be prevented, compared with expectant management until 41 weeks’ gestation.23 Our study findings support this recommendation for those with class II and III obesity, in particular. When accounting for gestational age, maternal weight gain and maternal diseases of pregnancy, pregnant people with a BMI of 30 or higher have been found to have 3.5 times the risk of stillbirth at 37–39 weeks’ gestation and 4.6 times the risk at 40 weeks’ gestation or more, compared with those with normal BMI.2
Yao and colleagues24 showed that the risk of stillbirth increased at 39 weeks’ gestation among pregnant people with obesity, compared with those with normal BMI. Nearly 25% of stillbirths that occurred between 37 and 42 weeks’ gestation were associated with obesity, although these findings were not adjusted for confounders. A later study from Yao and colleagues25 suggested that expectant management of pregnant people with obesity at term was associated with a higher risk of perinatal mortality, compared with those with normal BMI. However, in the latter study, obesity class II and III were not separately analyzed, other confounders were not controlled for and pregnant people with pre-existing hypertension and diabetes were excluded.
The reduction in risk of stillbirth that we saw at 39 weeks’ gestation among pregnant people with class II and III obesity may be attributed to delivery for anticipated development of pregnancy complications between 38–39 weeks’ gestation, with higher risk of stillbirth among those delivering beyond this time. Given the overall lower ORs for stillbirth among those with class III obesity compared with those with class II obesity, clinical anticipatory intervention may already be occurring for pregnancies in the highest obesity category. Further research evaluating different hospital practices around surveillance and delivery planning in this population is needed.
We anticipated a greater proportion of antepartum than intrapartum stillbirths in the higher BMI categories based on both the higher prevalence of comorbidities that increase the risk of antepartum stillbirth, as well as the proinflammatory state of obesity26 and its possible effects on placental function,27,28 maternal nutrition, and increased risk of antepartum rather than intrapartum complications. Given that increased BMI is often an indication for continuous fetal monitoring, more intrapartum stillbirths may have been prevented in this population. In terms of assessing antepartum stillbirth specifically, Stephansson and colleagues29 showed an approximate doubling in antepartum stillbirth for pregnant people with increased BMI compared with those with normal BMI; we also found more antepartum stillbirths but not to the same degree.
Limitations
Stillbirth is an uncommon outcome, and stratified analysis across subgroups of gestational age and BMI had limited power. We approximated the method described by Joseph and Kramer to calculate fetuses-at-risk, representing survival analysis from a fetal perspective, using a Cox model.30,31 One limitation of this method is that the same degree of risk is assumed across gestational age, which may not be true for our study population. We therefore also used a logistic regression model to generate point estimates for ORs for stillbirth, stratifying for gestational age, to circumvent the issue of proportional hazard assumptions, as well as small sample sizes within each gestational age grouping. We did not include data on race, ethnicity, immigration status and maternal education because these are incompletely captured in the BORN dataset. We did not have information specifying the gestational age at which pregnancy complications developed. Although BMI data were obtained from a combination of clinical assessment and self-report, self-reported and measured values for BMI have been shown to be highly correlated.32–34 A recent study found that the under-reporting of weight averages 2 lbs overall and about 5–7 lbs in the higher obesity classes, which, when converted to BMI, represents a small measurement error.35 For most people, this does not affect BMI categorization.34 Analysis of most of the covariates as dichotomous may also have imperfectly captured their influence on stillbirth risk. Data for BMI and covariates were missing in 12% and 15% of pregnancies, respectively, with more data missing in earlier years. Supplemental analysis showed that the study cohort and the cohort with missing data were not different for most of the covariates, suggesting that the data were likely missing at random rather than because of bias, limiting the impact of the missing values on our study findings. We did not restrict analysis to later years, given the relatively small sample size.
Conclusion
Our results showed that maternal obesity was associated with an increased risk of stillbirth, particularly at term. This association remained strong after partial adjustment for additional risk factors for stillbirth known to be prevalent in the obese population.
Acknowledgements
The authors would like to acknowledge Sara C.S. Souza, clinical research coordinator at the Ottawa Hospital Research Institute, and Donaldo Canales, research methodologist at Saint John Regional Hospital, for their contributions to the tables, graphing the analyzed data and formatting. They would also like to acknowledge Alysha Harvey, clinical research program manager at the Ottawa Hospital Research Institute, for her contributions to study organization, coordination and manuscript preparation.
Footnotes
Competing interests: Qun Miao reports funding from the Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation. Yanfang Guo reports funding from the CIHR and the Dalhousie University Advancement Funding Grant. Mark Walker sits on the board of the CHEO Research Institute. Laura Gaudet reports consulting fees from the Canadian Medical Protective Association. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Naila Ramji, Daniel Corsi, Monica Gad, Yanfang Guo, Natalie Rybak, Ruth Rennicks White, Mark Walker, and Laura Gaudet contributed to study concept and design. Naila Ramji, Daniel Corsi, Monica Gad, Sheryll Dimanlig-Cruz, Qun Miao, Shi Wu Wen, and Laura Gaudet contributed to data acquisition and analysis. Naila Ramji, Daniel Corsi, Monica Gad, Sheryll Dimanlig-Cruz, Qun Miao, Shi Wu Wen, Mark Walker, and Laura Gaudet contributed to data interpretation. Naila Ramji drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the Canadian Institutes of Health Research (no. MFM-146444) and the Dalhousie University Advancement Funding Grant. Laura Gaudet is supported by the Canadian Institute for Health Sciences. The funders were not involved in study design, analysis or interpretation of data, nor were they involved in writing of this manuscript nor the decision to submit the article for publication.
Data sharing: The data analyzed for this study are held securely at the prescribed registry, Better Outcomes Registry and Network (BORN) Ontario. Legal data sharing agreements between BORN Ontario and data providers (e.g., health care organizations and government) prohibit BORN Ontario from making the data set publicly available, owing to their containing information that could compromise the privacy of research participants. The analytic code may be available on request from the corresponding author, with the permission of BORN Ontario.
- Accepted January 2, 2024.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/