Varicella zoster virus has a worldwide distribution and is highly infectious; its spread is airborne via droplets or direct contact.
Primary varicella infection as an adult is associated with high morbidity and mortality.
Some countries do not have programs for routine vaccination against varicella; therefore, people from these countries are at risk of infection.
The skin lesions of varicella may be overlooked in patients with darker skin.
Review of vaccine records, including updating vaccination series, is an important component of immigrant health and preventing disease among people born outside Canada.
Patient A
A 50-year-old man presented to the emergency department with 3 days of acute severe chest pain that radiated to his back, subjective fever and decreased oral intake. He had a history of diabetes, hypertension and dyslipidemia, for which he was on metformin–sitagliptin, gliclazide, empagliflozin, perindopril–indapamide and rosuvastatin. He had been on methotrexate for more than 5 years to treat atopic dermatitis. On examination, he was afebrile, his oxygen saturation was 98% on room air and he had a papular skin rash on his trunk and extremities. He was admitted for further investigations and management.
The patient’s laboratory investigations are shown in Table 1. The initial computed tomography (CT) scan of his chest, abdomen and pelvis was unremarkable. We drew samples for blood cultures on admission, which grew methicillin-susceptible Staphylococcus aureus (MSSA). We started intravenous cloxacillin, and repeat blood cultures were sterile. Findings of a transthoracic echocardiogram were not suggestive of endocarditis. The hematology team proposed that his thrombocytopenia was caused by MSSA bacteremia. The patient had no features of thrombotic microangiopathy, and test results for HIV, hepatitis (A, B and C), cytomegalovirus and Epstein–Barr virus were negative.
Summary of laboratory investigations on admission
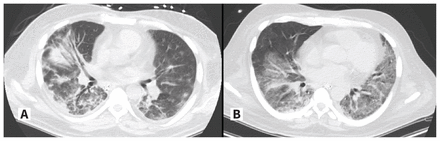
Two days after admission, the patient developed hypoxic respiratory failure with septic shock and was admitted to the intensive care unit (ICU) for vasopressor support and mechanical ventilation. A repeat CT scan of his chest showed confluent, multifocal, peribronchial, peripheral consolidation and ground glass opacities in the right lung, as well as ground glass opacities and nodules in the left lung (Figure 1A). We diagnosed community-acquired pneumonia with septic shock and started the patient on meropenem. His ICU stay was complicated by ventilator-associated pneumonia, acute renal failure and a reaction to cloxacillin, with eosinophilia and systemic symptoms. Despite appropriate antibiotics and supportive therapy, the patient remained dependent on the ventilator. Two weeks after his ICU admission, his brother was admitted to the ICU under similar circumstances (Figure 2).
Computed tomography scans of the chest of (A) a 50-year-old man (patient A) and (B) of a 52-year-old man (patient B), showing bilateral infiltrates related to primary varicella infection.
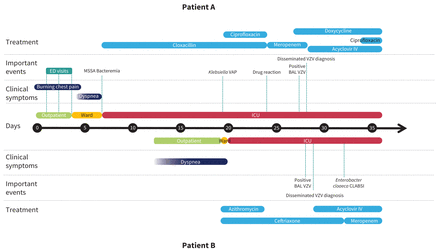
Timeline of events for a 50-year-old man (patient A) and 52-year-old man (patient B) with primary varicella infection. Day 0 is the date of first symptoms of patient A. Note: BAL = bronchoalveolar lavage, CLABSI = central line–associated bloodstream infection, ED = emergency department, ICU = intensive care unit, IV = intravenous, MSSA = methicillin-susceptible Staphylococcus aureus, VAP = ventilator-associated pneumonia, VZV = varicella zoster virus.
Patient B
A 52-year-old man presented to the emergency department with 1 week of progressive dyspnea. He had a history of diabetes with associated nephropathy, hypertension and dyslipidemia, for which he was on metformin, linagliptin, gliclazide, amlodipine–perindopril and rosuvastatin.
Within 24 hours of presentation, we admitted the patient to the ICU for acute hypoxic respiratory failure. Laboratory investigations are shown in Table 1. A CT scan of his chest showed multifocal ground glass opacities with areas of intralobular septal thickening, suggestive of infection (Figure 1B). We diagnosed community-acquired pneumonia.
We performed bronchoalveolar lavage on both patients. Samples from both were positive for varicella zoster virus (VZV) by polymerase chain reaction (PCR), with a negative respiratory viral panel (influenza A and B, respiratory syncytial virus, SARS-CoV-2). After these results, we carefully re-examined both patients and found crusted erosions on the face, trunk and upper extremities of both (Figure 3). Both tested positive for VZV by PCR from blood samples and swab samples of lesions. Serological testing with blood samples drawn from each patient on presentation, compared with samples drawn later during hospital admission, showed acute seroconversion of VZV immunoglobulin (Ig) G.
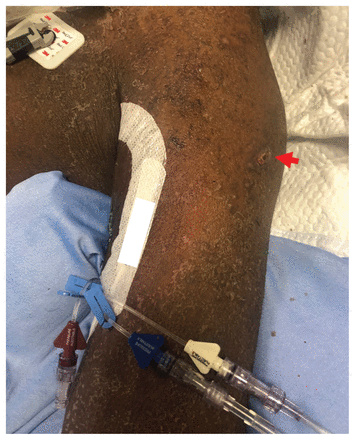
Photograph of a crusted erosion (arrow) on the upper left arm of a 50-year-old man (patient A), caused by a primary varicella infection. The background skin shows several brown, scaly, desquamating plaques secondary to a drug reaction with eosinophilia and systemic symptoms and his underlying chronic dermatitis.
We diagnosed primary VZV infection with disseminated disease and pneumonia in both patients, 28 days after patient A’s admission to hospital and 9 days after patient B’s admission. We started both on intravenous acyclovir. History obtained from others informed us that the brothers lived together, had immigrated to Canada from Fiji 2 decades earlier and had not travelled recently. They had never received diagnoses of primary VZV or been vaccinated against varicella.
We continued the intravenous acyclovir for about 4 weeks until the patients’ symptoms improved and their lesions were fully scabbed over. We discharged patient A on day 61. Patient B died 119 days after admission of complications of sepsis from hospital-acquired pneumonia.
Discussion
Primary VZV, also known as chickenpox, is a virus found worldwide.1,2 It is extremely contagious and is spread by direct contact with lesions or by inhalation of aerosols from vesicular lesions or infected respiratory tract secretions.1 Since the implementation of an effective public vaccination program for VZV in Canada, the rates of primary VZV infection, hospital admissions and death have decreased across all age groups.3 However, the age of primary infection has shifted to a later age owing to herd immunity.3 Adults born outside Canada may be particularly susceptible in the absence of routine VZV vaccination programs or different transmission dynamics in their country of origin.3
Clinical presentation, diagnosis and management
The spectrum of clinical illness varies according to patient age and immunity.1,2 Primary infection with VZV during childhood is usually a relatively benign and self-limiting illness in healthy children.1,2 In children, varicella infection often presents as mild fever and constitutional symptoms, which usually resolve several days after the onset of a generalized blister-like rash.
In contrast, primary VZV infection in adults is often associated with high morbidity and mortality.2–4 Complications are more common among adolescents and adults, pregnant people and immunocompromised people than among children; they include bacteremia from secondary skin and soft tissue infections, pneumonia, hepatitis and encephalitis.1,2,4,5 Case fatality rates are highest among adults.4,6
One of the most severe and life-threatening complications of disseminated disease is the development of varicella pneumonia, which occurs in 1 in 400 cases among immunocompetent adults and is associated with an overall mortality of 10%–30%.7 Respiratory symptoms, including dyspnea, cough and tachypnea occur 1–6 days into the course of the illness, in association with fever.1,7 Chest imaging often shows a bilateral nodular or interstitial pneumonia pattern.7 Patients with progressive respiratory failure requiring mechanical ventilatory support have mortality rates of 43%–50% despite maximal supportive measures.8
A diagnosis of VZV infection can be made clinically based on the characteristic vesicular lesions in all stages of development.9 Laboratory-confirmed diagnosis of VZV is made with PCR testing of vesicular skin lesions.9 Disseminated disease and complications are often diagnosed by the microbiological confirmation of cutaneous lesions with VZV PCR testing, in addition to a compatible clinical syndrome such as pneumonia or encephalitis. Cerebral spinal fluid, serum and bronchial washings can also be tested.9 Serological confirmation of an acute infection can be made by the seroconversion of VZV IgG.9
Acyclovir and its analogues (valacyclovir, famciclovir) are highly effective antiviral treatments for primary varicella. The decision to use antiviral therapy depends on the extent of infection and host factors.1,7 In immunocompetent adults, antivirals have a limited window of opportunity to affect the outcome of VZV infection, as most viral replication has stopped 72 hours after the onset of the rash. In contrast, this window is longer in immunocompromised hosts, as viral shedding in these patients can be prolonged. In patients who develop severe complications, including varicella pneumonia, clinicians should administer acyclovir (10 mg/kg) intravenously every 8 hours for 7–10 days, with the duration tailored to each patient’s clinical course.1,7 Apart from antiviral therapy, care is mainly supportive and mechanical ventilation may be required in patients with respiratory failure.7
Our 2 patients did not have a history of childhood primary VZV infection, and had immigrated in adulthood from Fiji, which does not have a varicella vaccination program. This made them susceptible to developing a primary varicella infection in adulthood. Their acute seroconversion of VZV IgG and disseminated skin rash (the importance of which was not initially recognized) were consistent with a primary varicella infection, which was diagnosed only after they had been in hospital for some time. Both brothers developed complications of primary varicella infection, including disseminated disease with varicella pneumonia and, in the case of patient A, bacteremia from a secondary bacterial skin and soft tissue infection. Targeted vaccination of adults who have immigrated from countries that do not provide immunization against VZV may be an effective strategy in preventing primary disease and its associated complications.3
In both patients, the diagnosis of primary varicella infection was made after a bronchoalveolar lavage identified VZV by PCR testing. Both underwent a repeat dermatological examination, which found crusted erosions suggestive of evolving varicella. One factor that likely contributed to the delayed diagnosis was the Fitzpatrick type 5 skin seen in both patients. Some physicians are unfamiliar with dermatological examination of darker skin, and poor interpretation of findings can lead to suboptimal care.10,11 Varicella has been classically described as presenting as erythematous papules that evolve into small clear vesicles with an erythematous halo that eventually form crusted erosions, but erythema is not as clearly visible on darker skin.10,11 In patients with darker skin, erythema may not be present or may have a more violaceous hue.11 Physicians may find it useful to use the tips of the fingers to assess for skin textural changes (e.g., in patients with atopic dermatitis).11 More variation in educational clinical images and improved teaching to clinicians are needed to increase competency in diagnosing skin disease in patients with darker skin.10
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All authors conceived the work. Jordan Mah, Anthony Lieu, Emma Heck and Alejandra Ugarte-Torres drafted the manuscript, All authors critically revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/