Endometriosis is a common disorder that can cause pelvic pain and infertility, may involve multiple organ systems and can adversely affect quality of life.
The cause of endometriosis is not fully understood, and no curative treatment currently exists.
Although surgery can provide a definitive histopathological diagnosis, most international guidelines now recommend a nonsurgical diagnosis based on symptoms, findings on physical examination and imaging to reduce delays to starting treatment.
Treatments target symptoms and include hormonal suppression, surgery or a combination of both approaches, as well as multidisciplinary care to address persistent pain arising from central sensitization and nociplastic pain processes, if required.
Endometriosis is a chronic condition defined by the presence of endometrial-like tissue outside of the uterus, which can lead to estrogen-driven inflammation. The extent of disease can be highly variable, ranging from minimal peritoneal deposits to deep disease that can invade into the bowel, bladder and ureter and, more rarely, spread to extrapelvic (e.g., cutaneous, thoracic) sites. Endometriosis is a complex disease that has considerable impact on the quality of life of those affected and that has no cure. It remains poorly understood. We review the epidemiology, pathophysiology, diagnosis and management of endometriosis, based on the best available evidence and clinical guidelines (Box 1).
Box 1: Evidence used in this review
We conducted a targeted, nonsystematic MEDLINE (1960 to January 2022) search using the term “endometriosis” on its own and with the AND function with the terms “pathophysiology,” “diagnosis,” “treatment,” “pain,” “infertility,” “surgery” and “medications.” We focused on research involving human adults, and on clinical guidelines, systematic reviews and randomized trials.
What is the burden of endometriosis?
Endometriosis is estimated to affect about 10% of women of reproductive age, and an unknown number of gender-diverse people.1 It is one of the most prevalent gynecologic conditions, believed to affect roughly 1 million people in Canada.1 Case series have identified endometriosis in 40%–50% of women and adolescents with persistent pelvic pain and in 30%–40% of women with infertility.2 The condition can cause severe dysmenorrhea, deep dyspareunia and chronic pelvic pain, as well as bowel and bladder symptoms and fatigue. Symptom severity is not correlated to extent of disease; patients with substantial disease may be asymptomatic, adding to the puzzle of this condition. 3 Endometriosis can involve multiple organ systems and its symptoms are often chronic, which can affect work productivity, social life, intimate relationships and mental health considerably, 4,5 and lead to substantial societal costs.6 Endometriosis also affects fertility by altering the peritoneal environment or by distorting the pelvic anatomy; about 30% of patients with endometriosis have difficulty conceiving.7
What causes endometriosis?
Many theories have been suggested to explain the development of endometriosis, but none are definitive. The most accepted theory is that endometrial cells reach the peritoneal cavity through retrograde menstruation (a physiologic process that occurs in 90% of women); these cells are usually broken down and cleared.8 Endometriosis is postulated to develop because of alteration in this process owing to factors such as cellular adhesion and proliferation, somatic mutations, inflammation, localized steroidogenesis, neurogenesis and immune dysregulation.9 The endometrial-like cells are able to implant outside the uterus and respond to estrogen stimulation from the ovaries and the cells themselves, leading to inflammation and subsequent scarring and adhesions. Other theories include coelomic metaplasia, whereby the normal peritoneal tissue (i.e., mesothelium) transforms via metaplastic transition to ectopic endometrial-like tissue. Hematogenous or lymphatic spread is postulated to explain extrapelvic endometriosis.
What are the known risk factors for endometriosis?
Risk factors for endometriosis include low birth weight, Mullerian anomalies, early menarche, short menstrual cycles, increased menstrual flow, low body mass index and nulliparity.10 People with endometriosis may have a genetic predisposition, with twin studies showing 50% heritability11 and epidemiologic studies showing 3–15 times increased risk of disease among first-degree relatives of patients with endometriosis.12 Racial and ethnic differences in the prevalence of diagnosed endometriosis have been reported; a systematic review found that Asian women had a higher risk, and Black women had a lower risk, of endometriosis than White women, but it is possible that these estimates reflect a bias related to access to care.13
What are the subtypes of endometriosis and their clinical manifestations?
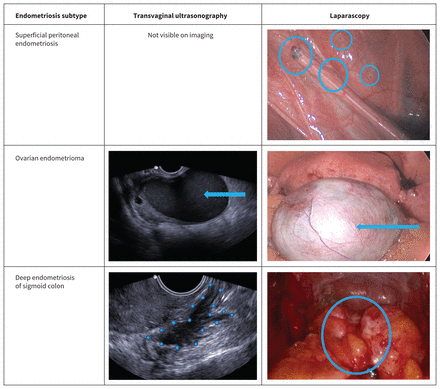
Three subtypes of pelvic endometriosis are important to recognize as they may affect symptom presentation and method of diagnosis (Figure 1). Superficial peritoneal endometriosis is the most common subtype and consists of lesions of various colours located on the surface of the peritoneum. Endometriomas are ovarian cysts that contain dark, blood-stained fluid (often called chocolate cysts). Deep endometriosis (previously called deep infiltrating endometriosis) is identified by lesions that extend beyond the peritoneum; these lesions are often nodular and fibrotic, and have the capacity to invade adjacent pelvic organs such as the rectosigmoid, ureter or bladder.14 Subtypes may overlap; some patients may have more than 1 manifestation of the disease concurrently. Endometriomas frequently co-occur with deep endometriosis, and finding an endometrioma on ultrasonography should prompt further investigation, especially if the patient reports severe pain.15 Deep endometriosis has the capacity to cause end-organ damage such as kidney failure (from ureteric obstruction) or bowel obstruction, so timely diagnosis and management is important. Extrapelvic disease is a less common clinical presentation of endometriosis and may occur at sites such as the diaphragm, thoracic cavity and surgical scars.
Imaging and laparoscopic appearance of endometriosis subtypes.
Symptoms of endometriosis can vary and may change over time. Endometriosis is sometimes diagnosed incidentally at surgery performed for other indications, with the patient having reported no symptoms. Regardless of subtype, most (90%) symptomatic patients have secondary dysmenorrhea, which can be incapacitating and is often their presenting problem.16 This can be distinguished from primary dysmenorrhea, which is usually shorter in duration and responds well to nonsteroidal anti-inflammatory drugs. Deep dyspareunia (hitting pain, felt in the upper vagina during sexual intercourse), chronic pelvic pain and infertility are also common symptoms and can coexist with dysmenorrhea. Less commonly, any of these 3 symptoms can be the main presenting problem without the presence of dysmenorrhea. Deep endometriosis that invades adjacent organs can lead to symptoms at time of menstruation such as painful bowel movements (dyschezia), bloody stools (hematochezia), dysuria or hematuria.
Diaphragmatic or thoracic implants can cause cyclical dyspnea, chest or shoulder pain, hemoptysis and pneumothorax.17 Other symptoms that are less specific but are frequently reported by patients with endometriosis include abdominal pain and bloating, abnormal uterine bleeding, low back pain and fatigue. Given this heterogeneous symptomatology, it is important for health care providers to have a high index of suspicion for endometriosis.
What is the natural history and prognosis of endometriosis?
The natural history of the disease was observed using laparoscopy, repeated at 6–12 months, among patients enrolled in the untreated arms of 2 randomized trials that evaluated surgical treatment of patients with minimal to moderate disease. Endometriosis progressed in 29%–45% of patients, was unchanged in 33%–42% of patients and regressed in 22%–29% of patients.18,19 This information changed the long-held belief that endometriosis is always progressive.
Most patients report that their symptoms started in adolescence and improve at menopause, although some patients continue to have pain after menopause.20 The improvement at menopause is likely owing to lack of estrogen stimulation.
Although current medical and surgical therapies are not curative, they provide considerable symptom relief for many patients. However, some people with endometriosis develop a more complex, persistent pain problem despite complete treatment, which may be secondary to central sensitization or nociplastic pain, recently defined by the International Association for the Study of Pain as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain.”21 Mechanisms of central sensitization in endometriosis include a reduction in descending modulation of peripheral signals (gate theory) and cross-sensitization that gives rise to symptoms in visceral and somatic structures (via viscero–visceral and viscero–somatic cross-talk in the spinal cord).22 The development of central sensitization may account for the evolution of cyclical pain to chronic pelvic pain and the development of other chronic pain conditions. In 2015, the National Institutes of Health recognized the entity of chronic overlapping pain conditions as a cluster of chronic pain conditions that often co-exist, occur predominantly in women, and likely share common immune, neural and endocrine mechanisms. 23 Endometriosis was one of these conditions, along with commonly co-existing conditions, such as vulvodynia, irritable bowel syndrome and painful bladder syndrome. Other chronic overlapping pain conditions are chronic migraine, chronic low back pain, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia and temperomandibular disorders.23 Patients who do not respond, or have only a short-term response, to endometriosis-targeted treatments and who have concurrent pain conditions may have developed a central sensitization or nociplastic pain process.24,25
Evidence suggests that early treatment of endometriosis and associated pain may decrease the risk of development of chronic pain, which further supports the importance of early assessment and intervention.22
How is endometriosis diagnosed?
Despite research on biomarkers, no blood test reliably diagnoses endometriosis. Laparoscopic visualization of endometriosis lesions with histopathological confirmation has long been considered the gold standard for diagnosis, but recent guidelines advocate a nonsurgical (clinical) diagnosis based on symptoms and findings on physical examination and imaging.26,27 This change has resulted from the recognition that surgery is not considered curative and has risks, and that reliance on a surgical diagnosis can lead to an unacceptably long delay (up to 11 yr) between onset of symptoms and start of adequate treatment.28,29 Other factors contribute to a delay in diagnosis, including symptom variability, suboptimal health provider and patient awareness and knowledge of this condition, stigma around discussing gynecologic symptoms and societal normalization of women’s pain.10,30 To overcome some of these factors, health care providers should routinely ask patients about their menstrual cycle and about endometriosis-associated symptoms and their impact on quality of life. Diagnosis in adolescents may be particularly difficult, as acyclic pain is more common in this population.
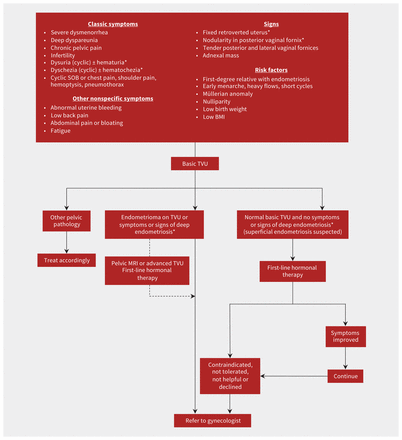
The history and physical examination are essential to making a diagnosis of endometriosis (Figure 2). A case–control study from the United Kingdom of more than 5000 patients with endometriosis found that they were more likely than controls to have dysmenorrhea (odds ratio [OR] 8.1), dyspareunia or postcoital bleeding (OR 6.8), abdominopelvic pain (OR 5.2), menorrhagia (OR 4.0) and a history of subfertility (OR 8.2).31 Table 1 reviews the differential diagnoses of the most common symptoms of endometriosis — dysmenorrhea, deep dyspareunia and chronic pelvic pain — with their salient clinical features; however, these conditions can also coexist with endometriosis.
Diagnosis and first-line management of endometriosis. Note: BMI = body mass index, MRI = magnetic resonance imaging, SOB = shortness of breath, TVU = transvaginal ultrasonography. *Suspect deep endometriosis.
Differential diagnosis of the most common symptoms of endometriosis
The pelvic examination can be very uncomfortable for a patient with pelvic pain symptoms, and may not be possible in some circumstances. It should be undertaken with informed consent and conducted in a gradual manner based on tolerance to each step (single digit, then bimanual, then speculum), with frequent check-ins with the patient. The examination may reveal adnexal masses in patients with endometrioma, or a fixed retroverted uterus or palpable firm nodule of the posterior vaginal fornix (corresponding to the posterior cul-de-sac of the pelvis) in patients with deep endometriosis. Sometimes, posterior vaginal fornix nodules can be visualized on speculum examination, often with a bluish tinge. Tenderness of the posterior vaginal fornix (corresponding to the uterosacral ligaments) or lateral vaginal fornices (corresponding to the adnexae) can be found in patients affected by endometriosis. The clinical examination has low diagnostic accuracy, so a normal examination does not rule out endometriosis.26,32 The examination may help with detecting other potential causes of pelvic pain, such as pelvic floor tenderness (pelvic floor myalgia), or bladder base tenderness (painful bladder syndrome) (Table 1).
Imaging is an important modality for the nonsurgical diagnosis of endometriomas and deep endometriosis. Transvaginal ultrasonography, an inexpensive and readily available test, is recommended as a first-line investigation for patients suspected of having endometriosis.26,27 Basic transvaginal ultrasonography, as performed in most ultrasound units, can be used to diagnose endometriomas with high accuracy and can also rule out other pelvic pathology. Advanced transvaginal ultrasonography — which incorporates the sliding sign between uterus and sigmoid colon, and examination of the anterior and posterior compartments for endometriosis nodules — has been shown to reliably detect deep endometriosis in systematic reviews.33 This type of ultrasonography is performed by sonographers, radiologists or gynecologists with a special interest and training in endometriosis imaging but is not routinely available in many regions in Canada. Criteria for performing and reporting transvaginal ultrasonography for patients with suspected endometriosis have been published, and will hopefully be adopted by all ultrasonography units.34 If pelvic examination or transvaginal ultrasonography are not possible or acceptable to the patient, transabdominal or transrectal ultrasonography can be performed instead.
Magnetic resonance imaging can be used to diagnose deep endometriosis and has similar sensitivity and specificity (> 90%) to advanced transvaginal ultrasonography, although its accuracy is affected by the protocols used and the experience of the reader.33 Both modalities are excellent at detecting adenomyosis, a condition that commonly co-occurs with endometriosis, and is also a cause of severe dysmenorrhea. Providers may need to communicate with their local radiologist to find out which imaging modality is most readily available in their region for detection of deep endometriosis.
No imaging modality can reliably detect superficial peritoneal endometriosis; it may be suspected based on symptoms suggesting endometriosis and tenderness of the vaginal fornices on pelvic examination (Figure 2). A definitive diagnosis can be made only at surgery, but current guidelines recommend against performing a laparoscopy for diagnostic purposes alone.26,35
The current recommendation to provide a clinical diagnosis of endometriosis based on symptoms, signs and imaging, without the need for pathological confirmation, is important because this approach facilitates validation of symptoms, empowers providers to start treatment early and provides information to patients about their health, allowing them to make more informed choices about their treatment. Providing first-line treatment on the basis of a clinical diagnosis also reduces delays in treatment and, thus, decreases the likelihood of long-term sequelae of the disease.
How should endometriosis be managed?
Options for the treatment of patients with symptomatic endometriosis are hormonal therapies that suppress ovulation and menstruation, surgical treatment or a combination of both.35 Diet and lifestyle modifications may also be helpful but have not been well studied.36 Diets that target concurrent conditions such as irritable bowel syndrome and painful bladder syndrome have more evidence supporting their usefulness.37,38
Nonspecialist health care providers should feel empowered to diagnose endometriosis and start management (Figure 2). Nonsteroidal anti-inflammatory drugs may be a helpful first-line treatment for symptoms of dysmenorrhea, but no evidence suggests that they improve nonmenstrual symptoms.39 Many hormonal options can be used to treat endometriosis; all have a comparable efficacy of 60%–80%, and are recommended by clinical practice guidelines (Table 2).26,27,35 However, they have variable costs and adverse effects.40 The goal of hormonal therapy is to suppress the menstrual cycle, create amenorrhea and, preferably, stop ovulation if that process is painful. Hormonal therapies are contraceptive and, therefore, are not appropriate for patients who are trying to conceive. Nonhormonal medical therapies that target inflammatory or angiogenic pathways are being explored, but none are currently available.40
Hormonal therapies for endometriosis
Hormonal suppression can be achieved with combined estrogen–progestin contraceptives (cyclic or continuous, with the latter being more effective) or progestin-only medications (oral or injectable medications, subcutaneous implants or intrauterine devices). Evidence supports their effectiveness for endometriosis symptoms, and current guidelines consider all these as acceptable first-line options. Two systematic reviews and a Cochrane review (including 5 randomized controlled trials [RCTs]) have concluded that treatment with combined hormonal contraceptives reduces endometriosis-associated pain — including dysmenorrhea, noncyclic pelvic pain and dyspareunia — and improves quality of life, compared with placebo. However, these reviews also noted that the studies were of low quality, with a high risk of bias and short follow-up duration (3–11 mo).41–43 The efficacy of various progestogens was evaluated in a Cochrane review and a systematic review focused on dienogest.44,45 They found that continuous progestogens are effective for the treatment of endometriosis-associated pain, with variable adverse effects and no evidence that 1 oral progestogen is more efficacious than another. In a systematic review comparing the levonorgestrel-releasing intrauterine system with gonadotropin-releasing hormone (GnRH) agonists, which included 5 RCTs, both were comparable in relieving endometriosis-associated pain.46
Patient-centredness should underpin choice of treatment and discussions should include information on individual risk factors and patient preferences. Several treatments may need to be tried before one is found that provides good cycle suppression with acceptable adverse effects. Once a successful first-line treatment is found, it can be continued for many years.
Second-line therapies include GnRH agonists and antagonists, as well as aromatase inhibitors. Use of GnRH agonists and, at higher doses, GnRH antagonists requires add-back hormone replacement therapy to counteract the menopausal adverse effects of severe hypoestrogenism. Use of oral danazol, a synthetic androgen, is no longer supported, given its adverse effects. Second-line treatment options are usually started by a gynecologist, most often when endometriosis is confirmed by imaging or surgery. Long-term use of second-line agents is sometimes required and, therefore, ongoing administration may be provided by the primary care provider. The available hormonal therapies, their adverse effects and relative costs are listed in Table 2.
Surgical treatment is offered when drug therapies are contraindicated (such as for patients who are trying to conceive), are not tolerated or have failed to provide adequate relief. A minimally invasive approach with complete treatment of the disease is considered best practice by most international guidelines. 26,27,35 Some patients may choose surgery as their first option after counselling about its benefits (including fertility benefits, which are affected by factors such as age) and its risks and limitations, including recurrence of disease and persistence of pain from other causes.26,27,35 In patients for whom endometriosis has led to ureteric or bowel obstruction, surgery may be the only management option.
If surgical management is pursued, endometriosis is usually staged according to the revised American Society for Reproductive Medicine (ASRM) classification system as minimal, mild, moderate or severe (Stages I–IV). This staging system reflects extent of disease and anatomic distortion, and correlates with surgical complexity, but is poorly correlated to severity of pain and fertility.10
The Endometriosis Fertility Index, a tool that combines patient history, revised ASRM staging and anatomic state of the adnexae at the end of surgery, has been shown to be reliable in predicting the likelihood of conceiving without in vitro fertilization after surgery.47 In the context of infertility, surgery for treatment of superficial peritoneal endometriosis or endometriomas may improve the chance of natural conception, but must be balanced with other options such as assisted reproductive technologies.26
A Cochrane systematic review concluded that surgery was effective for pain symptoms but included only 3 small RCTs with follow-up of 6–12 months.48 Other systematic reviews have shown a persistence or recurrence rate of 22% at 2 years and of 40%–50% at 5 years after surgery.49 Treating patients with hormonal management postoperatively may decrease the rate and speed of recurrence of pain symptoms.50 Because of the complexity and higher risks associated with surgery for patients with deep endometriosis, detection of deep endometriosis on imaging allows for improved surgical planning and timely referral to specialized surgeons or centres of expertise. Unfortunately, access to such care is limited in some regions of Canada.
Laparoscopic hysterectomy, with or without removal of 1 or both ovaries, may also be an option for select patients — such as those who have ongoing dysmenorrhea or heavy menstrual bleeding, adenomyosis or recurrence of disease and who have no desire for future fertility — after appropriate counselling of benefits and risks. Hysterectomy with concurrent treatment of endometriosis has better pain outcomes than conservative surgery alone, but it is still not curative.51 Removal of both ovaries causes premature surgical menopause with potential adverse effects on bone and heart health (as compliance with hormone replacement therapy is low) and provides only marginal additional benefit for pain over hysterectomy alone.52
Some patients may not respond to medical or surgical management and may develop persistent pelvic pain that may reflect central sensitization or nociplastic pain, with accompanying chronic overlapping pain conditions. In patients with complex pain, a multidisciplinary plan of care that follows chronic pelvic pain guidelines is most likely to lead to improved quality of life. This may include pain education, pelvic physiotherapy, psychological interventions (such as cognitive behavioural therapy, acceptance and commitment therapy or mindfulness-based therapy) and targeted interventions for other pain contributors. 24,53,54 A multidisciplinary, multimodal, patient-centred approach has been recognized as best practice for chronic pain conditions. Primary care providers often play a central role in coordinating this care or referring the patient to a specialized clinic, where available.25,55
Who should be referred to a gynecologist?
If a patient has symptoms and signs of deep endometriosis or investigations reveal an endometrioma, they should be referred for assessment by a gynecologist, who will likely order further imaging with pelvic magnetic resonance imaging or advanced transvaginal ultrasonography. Depending on the wait times for specialist consultation or imaging, it may be appropriate to seek both at the same time and start first-line medical therapy. Patients with suspected superficial peritoneal endometriosis who do not respond to, have contraindications to or decline first-line medical management options, and those who are actively trying to conceive or have infertility, would also benefit from gynecologic assessment and management (Figure 1).
Conclusion
Endometriosis is a common and complex condition that can cause considerable distress and can lead to the development of chronic pelvic pain, infertility or end-organ damage. Early recognition and diagnosis are key to providing timely treatment. Primary care providers can make a clinical diagnosis of endometriosis and start first-line medical management. Referral to a gynecologist for second-line hormonal therapy or surgery is important, when indicated. Hormonal or surgical treatments can provide symptom relief and are part of a long-term management plan for this chronic condition. Multidisciplinary care may be required to address complex persistent pain.
Footnotes
Competing interests: Catherine Allaire reports conference support from Hologic and participation on advisory boards with AbbVie and Pfizer. She is a board member with the Canadian Society for the Advancement of Gynecologic Excellence, the International Pelvic Pain Society and the World Endometriosis Society. Mohamed Bedaiwy reports funding from the Canadian Institutes of Health Research (CIHR) and the Ferring Women Health Research Institute, royalties from AbbVie and Baxter, consulting fees from AbbVie and participation with the board of the Canadian Fertility and Andrology Society. Paul Yong reports funding from CIHR, Michael Smith Health Research BC, the Women’s Health Research Institute, the University of British Columbia and the International Society for the Study of Women’s Sexual Health. He reports meeting support from the Canadian Society for the Advancement of Gynecologic Excellence, the International Society for the Study of Women’s Sexual Health, the International Pelvic Pain Society and the World Endometriosis Society. He also sits on the data safety monitoring board a for CIHR-funded clinical trial, the strategic advisory board for the Women’s Health Research Institute, the boards of the Canadian Society for the Advancement of Gynecologic Excellence, the International Society for the Study of Women’s Sexual Health and the World Endometriosis Society. No other competing interests were declared.
This article was solicited and has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/