Abstract
Background: Rates of gestational diabetes are reported to be increasing in many jurisdictions, but the reasons for this are poorly understood. We sought to evaluate the relative contribution of screening practices for gestational diabetes (including completion and methods of screening) and population characteristics to risk of gestational diabetes in British Columbia, Canada, from 2005 to 2019.
Methods: We used a population-based cohort from a provincial registry of perinatal data, linked to laboratory billing records. We used data on screening completion, screening method (1-step 75-g glucose test or 2-step approach of 50-g glucose screening test, followed by a diagnostic test for patients who screen positive) and demographic risk factors. We modelled predicted annual risk for gestational diabetes, sequentially adjusted for screening completion, screening method and risk factors.
Results: We included 551 457 pregnancies in the study cohort. The incidence of gestational diabetes more than doubled over the study period, from 7.2% in 2005 to 14.7% in 2019. Screening completion increased from 87.2% in 2005 to 95.5% in 2019. Use of 1-step screening methods increased from 0.0% in 2005 to 39.5% in 2019 among those who were screened. Unadjusted models estimated a 2.04 (95% confidence interval [CI] 1.94–2.13) increased risk of gestational diabetes in 2019 (v. 2005). This increase was 1.89 (95% CI 1.81–1.98) after accounting for the rise in screening completion and 1.34 (95% CI 1.28–1.40) after accounting for changes in screening methods. Further accounting for demographic risk factors (e.g., age, body mass index, prenatal care) had a small impact (increase of 1.25, 95% CI 1.19–1.31).
Interpretation: Most of the observed increase in the incidence of gestational diabetes was attributable to changes in screening practices (primarily changes in screening methods) rather than changing population factors. Our findings highlight the importance of understanding variation in screening practices when monitoring incidence rates for gestational diabetes.
Rates of gestational diabetes are reported to be increasing worldwide.1 In Canada, the rate of gestational diabetes increased from 4.0% of deliveries in 2004 to 7.0% in 2014.2 In the United States, the rate increased from 4.8% in 2011 to 6.4% in 2019.3 Although some have speculated that the rise in gestational diabetes relates to changing demographics and lifestyle — such as increasing maternal age, changes in racial or ethnic composition of the population, decreased physical activity and poor diet quality — increases have been observed in all racial and ethnic groups after adjusting for increases in maternal age over time.3,4 Reasons for the continued increase in gestational diabetes remain poorly understood.
A diagnosis of gestational diabetes is dependent on completion of an antenatal glucose screening test. Therefore, increased uptake of screening could lead to a rise in diagnoses of gestational diabetes. Further, consensus is lacking on which method should be used to screen for gestational diabetes,5,6 and guidelines and diagnostic thresholds have changed in recent years.7 In 2010, the International Association of Diabetes in Pregnancy Study Groups (IADPSG) released a new guideline recommending a 1-step screening method (a single, 2-hour, 75-g oral glucose tolerance test).8 In Canada, national guidelines were updated in 20139 and 2016,10 in response to the IADPSG 2010 guideline.7 However, the 2013 and current national guidelines recommended a 2-step screening method (a 1-hour, 50-g glucose challenge test, followed by a 2-hour, 75-g oral glucose tolerance test for patients who screen positive), with the IADPSG 1-step method as an alternate approach.11 As 1-step screening is more sensitive than the 2-step approach, increased use of this method could be expected to increase the rate of diagnosis of gestational diabetes. 1 The advantage of 1-step screening is that only 1 laboratory visit is needed, but this single visit takes several hours, and requires fasting and 3 blood samples. Two-step screening begins with a less involved initial test (1 blood sample, nonfasting) but requires a second laboratory visit if the first test result is positive.
Population-level patterns of completion and methods of screening for gestational diabetes are poorly characterized because this information is not typically recorded in perinatal data registries or hospital discharge summaries. Therefore, the extent to which changes in completion or method of screening for gestational diabetes could explain the increasing risk of gestational diabetes remains unknown. However, a validation study recently showed that population-level billing records from a provincial laboratory can be used to accurately describe completion and method of screening for gestational diabetes.12 Using this data source, we aimed to evaluate the relative contributions of changes in screening completion, screening method and population-level characteristics to the rising risk of gestational diabetes in British Columbia, Canada.
Methods
Study design, data sources and study population
We identified a retrospective cohort of births at, or beyond, 28 weeks’ gestation in BC, Canada, using the population-based BC Perinatal Data Registry. This is a validated, chart-abstracted registry of all pregnancies in BC that result in a live birth or stillbirth at 20 weeks’ gestational age or later, or of an infant weighing at least 500 g at birth.13 We included all pregnancies with a first glucose tolerance test screening date between July 1, 2004, and June 30, 2019 (or in the seventh month of gestation, if unscreened). Birth registry records14 were linked with laboratory insurance billing records (BC Medical Services Plan),15 vital statistics data16 and census-derived data by Population Data BC,17 using personal health numbers and other unique identifiers. We excluded pregnant people with pre-existing diabetes, late antenatal care (after seventh month) and inactive public insurance status for more than 10% of the pregnancy (by month).17 We excluded pregnancies with only a random glucose test or a hemoglobin A1c test as these tests were not indicated for gestational diabetes screening during the study period.
Measures
We defined a diagnosis of gestational diabetes by the presence of code O24.8 (from the Canadian version of the International Classification of Diseases and Related Health Problems, 10th Revision) in the discharge summary of the delivery hospital admission or from the BC Perinatal Data Registry (sensitivity and specificity both > 99%).13,18 Our team has recently shown that laboratory billing records have excellent validity in establishing completion and method of screening for gestational diabetes (> 92% sensitivity and > 99% specificity for both), compared with a gold standard of medical chart abstraction.12
We defined screening completion as completion of a recommended gestational diabetes test — either the 1- or 2-step approach.8 We classified patients who completed only a 50-g test (i.e., the first step of a 2-step screening approach) as having undergone a 2-step approach. Before October 2010, the 2-step diagnostic test available in BC was a 3-hour, 100-g oral glucose tolerance test, using Carpenter–Coustan diagnostic thresholds. 11,19 However, in October 2010, BC policy changed to recommend a 1-step screening with a 75-g glucose test according to IADPSG criteria for all pregnancies.11,20 Thereafter, both 1-step and 2-step screening methods used the 75-g diagnostic test.
We examined population characteristics that have been previously associated with risk of gestational diabetes, including parity, age at delivery, pre-pregnancy body mass index (BMI), multifetal pregnancy, a composite of pre-existing medical or obstetric conditions (any of pre-existing hypertension, pregnancy-complicating conditions or diseases, previous stillbirth, previous neonatal death or congenital anomaly in a previous birth), mother or birthing person’s region of birth, antenatal care by a midwife and inadequate prenatal care, as defined by the Adequacy of Prenatal Care Utilization Index.13,21,22 We classified the region of birth for the mother or birthing person based on the country indicated on the infant’s birth certificate (Asia; Canada, USA or missing; or other).
Statistical analysis
We estimated temporal trends in risk of gestational diabetes using generalized linear regression with a modified Poisson binomial approach.23 We built 4 nested models, and sequentially adjusted for potential determinants of gestational diabetes to characterize the extent to which they explained the crude change in risk. Model 1 estimated the crude temporal trend, adjusted for each calendar year as an indicator (dummy) variable. Model 2 adjusted for calendar year and screen completion (screened v. unscreened). Model 3 adjusted for calendar year, screen completion and method (2-step v. 1-step). Model 4 adjusted for calendar year, screen completion, method and population characteristics.4
We used model coefficients to predict yearly risk with 95% confidence intervals (CIs), with all characteristics fixed at 2005 mean levels, and compared these yearly risks to the observed 2005 risk (model 1) by calculating risk ratios (RRs). Thus, if a model explained all of the year-to-year variability in risk of gestational diabetes, we would expect no increase in the predicted risk for each year relative to the risk observed for 2005 (the baseline year).
We conducted sensitivity analyses to explore the robustness of our findings to different baseline and end years, in case the rates in these years were unusually high or low. We also adjusted for additional population characteristics (i.e., neighbourhood income quintiles, rural or urban status, history of macrosomia and ≥ 2 previous cesarean births) and stratified by health region. We hypothesized that individuals with a previous diagnosis of gestational diabetes would be more likely to be rescreened in a subsequent pregnancy using the more sensitive 1-step method, and that the proportion of individuals with a previous diagnosis increased over time. Therefore, we also adjusted for history of gestational diabetes (classified as previous pregnancy with a diagnosis of gestational diabetes, previous pregnancy with no diagnosis of gestational diabetes or no previous pregnancy in the database). We considered this as a sensitivity analysis since our ascertainment of the history of gestational diabetes was limited to a within-cohort assessment of repeated pregnancies. Specifically, if a previous pregnancy was not captured in our data (e.g., a pregnancy outside BC) or if the previous pregnancy occurred before our study time period, then we were unable to ascertain history of gestational diabetes. We also compared characteristics of people excluded because of inactive insurance status with those in the final analytic cohort. For additional sensitivity analyses, we imputed missing data for pre-pregnancy BMI and stratified by pre-pregnancy BMI and age categories (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221404/tab-related-content).24
Ethics approval
This study was approved by the University of British Columbia Research Ethics Board (#H20–00741).
Results
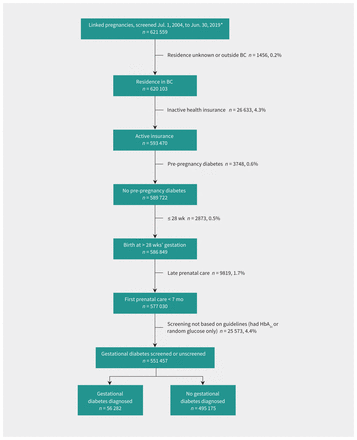
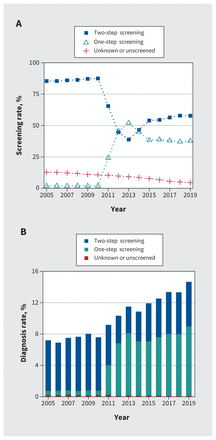
Of 621 559 pregnancies during the study time period, we included 551 457 pregnancies in the study cohort (Figure 1). Diagnoses of gestational diabetes increased from 7.2% in 2005 to 14.7% in 2019 (Figure 2 and Table 1). Screening completion increased from 87.2% in 2005 to 95.5% in 2019. The use of 2-step and 1-step screening methods changed over the study period. Among screened pregnancies, use of 1-step screening increased from 0.0% in 2005 to 50.5% in 2012, then decreased to 39.5% in 2019. Some population characteristics changed from 2005 to 2019. The proportion of pregnant people with a pre-pregnancy BMI higher than 30 kg/m2 increased from 8.1% to 12.8%; the proportion of those aged 35 years or older at delivery increased from 22.0% to 29.2%. Medical or obstetric complications increased from 4.3% to 7.0%, the proportion of pregnant people born in Asia increased from 21.6% to 26.9% and the use of antenatal midwifery care increased from 3.7% to 26.3%.
Population characteristics and exclusions for a study of gestational diabetes in British Columbia, Canada, 2005–2019. *All pregnancies (linked by valid personal identifiers in both the British Columbia Perinatal Data Registry and in BC Medical Services Plan billings) with nonmissing Medical Services Plan billings for that pregnancy and where the first glucose tolerance test date was between July 1, 2004, and June 30, 2019 (or in the seventh month of gestation, if unscreened).
Rates of gestational diabetes screening and diagnosis by screening method in British Columbia, Canada, 2005–2019.
Screening and diagnosis of gestational diabetes, and population characteristics for pregnant people in British Columbia, Canada (> 28 weeks’ gestation at delivery and without pre-existing diabetes) for selected years between 2005 and 2019
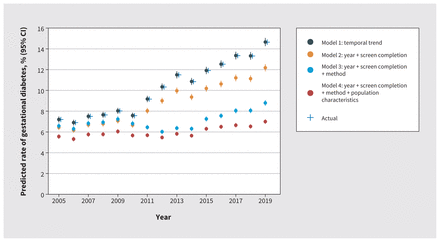
Our crude model estimated a 2.04-fold (95% CI 1.94–2.13) increase in risk of gestational diabetes for 2019, compared with 2005 (Figure 3 and Table 2). After accounting for the increase in screening completion over time (model 2), the risk of gestational diabetes in 2019 remained 1.89-fold (95% CI 1.81–1.98) higher. After accounting for screening method (model 3), the risk in 2019 was attenuated to a 1.34-fold (95% CI 1.28–1.40) increase. Further adjustment for trends in population characteristics had only a modest impact on the increased risk of gestational diabetes in 2019 compared with 2005 (1.25, 95% CI 1.19–1.31).
Predicted rates (with 95% confidence intervals [CIs]) of diagnosis of gestational diabetes after sequentially adjusting for screening completion, screening method and population characteristics in British Columbia, Canada, 2005–2019. Actual rate represents the annual diagnosis rate of gestational diabetes.
Estimated risk of gestational diabetes in each study year, compared with 2005, in a population-based cohort from British Columbia, Canada (> 28 weeks’ gestation at delivery and without pre-existing diabetes)
Results were unchanged in sensitivity analyses. The predicted effects of screening and population demographics on annual risk were generally similar for all nested models with different start years (2006, 2009) and end years (2018). Models with imputed data for BMI (missing in 26% of patients) resulted in similar estimates as the primary analysis. Adding adjustments for additional demographic and obstetric risk factors did not change model estimates (Appendix 1, Supplemental Table 1). Models stratified by health region, pre-pregnancy BMI and age showed similar patterns to the full cohort (Appendix 1, Supplemental Figures 2–4), although baseline risks differed by subgroup. Adding adjustments for history of gestational diabetes in a previous pregnancy explained most of the remaining increase in risk (1.1-fold increase from 2005 v. 2019, 95% CI 1.1–1.2) (Appendix 1, Supplemental Figure 3).
Interpretation
In this population-based cohort from BC, Canada, the increase in patients given diagnoses of gestational diabetes from 7.2% in 2005 to 14.7% in 2019 was explained primarily by changes in screening method and not by temporal changes in population characteristics. Our crude model of temporal trends estimated a 2.04-fold increase in gestational diabetes for 2019, compared with 2005. This temporal trend was attenuated to a 1.89-fold increase after accounting for the rise in screen completion that occurred during this time, and to 1.34-fold increase after accounting for screening method. Despite concerns that a higher proportion of pregnant people with high BMIs, older maternal age or risk factors for poor obstetric outcomes were leading to higher population-level rates of gestational diabetes, these were not important contributors to the annual increases in risk of gestational diabetes in our cohort. After accounting for completion and method of screening, further controlling for all population factors only modestly attenuated the increase, to a 1.25-fold increase.
Recent studies have evaluated trends in gestational diabetes worldwide.3,4,25–28 In jurisdictions where screening methods were unchanged, risks of gestational diabetes were relatively stable or increases were explained by population changes (e.g., BMI, age, ethnicity).25,26 For example, a study in China reported a relatively stable (17%) incidence of gestational diabetes from 2011 to 2018 after adjusting for age and BMI in a population where all individuals were screened with a 1-step, 75-g method. In jurisdictions with variable screening practices, rates of diagnosis doubled or tripled over time, even when controlling for population changes.3,4,27 These increases persisted across subgroups.3,29 For example, a recent US study of more than 12 million birth registration records found an increasing age-adjusted incidence of gestational diabetes, from 4.8% in 2011 to 6.3% in 2019.3 These increases were observed across all racial and ethnic groups. Although the authors speculated that observed increases could reflect changes in diagnostic criteria and screening, they were unable to examine this empirically owing to lack of screening data. The much lower incidence of gestational diabetes in the US compared with BC likely also reflects the uptake of the Carpenter–Coustan criteria from the American College of Obstetricians and Gynecologists guideline, which have a higher diagnostic threshold than the current Canadian guideline.30,31
Our findings highlight the importance of having data on screening methods, diagnostic criteria and screening completion to monitor trends and outcomes of gestational diabetes. The preferred and alternate screening methods described in the current Canadian guideline use different diagnostic criteria and will result in differences in diagnostic risk.7 Furthermore, studies should also consider the timing of changes in screening practice, such as the change from using a 3-hour, 100-g diagnostic test with Carpenter–Coustan criteria to using a 75-g diagnostic test (as per the Diabetes Canada guideline), which has a lower diagnostic threshold.9 After 2 Canada-wide revisions of guidelines for screening, diagnosis and treatment of diabetes in pregnancy, little research has been published on the impacts of these new guidelines on health systems across the country. British Columbia had the highest rate of gestational diabetes in 2017 at 13.9% among Canadian jurisdictions, which was significantly higher than the Canadian average at 9.0% and all other provinces (excluding Quebec).32 It is possible that uptake of 1-step screening differs in BC than in other provinces. For example, 1-step screening was completed by less than 1% of patients screened in Alberta from 2008 to 2012, and by 10% of those screened in northwestern Ontario from 2010 to 2017.33,34 Our study highlights the need for additional research to understand patterns of screening for gestational diabetes across Canada.
Our findings should be considered in light of the results of recent randomized controlled trials of screening methods for gestational diabetes and perinatal outcomes.35–37 Combining results from randomized controlled trials and large, well-conducted observational studies, a recent meta-analysis found equal rates of neonates who were large for gestational age, despite a twofold increase in diagnosis of gestational diabetes with 1-step screening and subsequent treatment.38 As the authors noted, although it appears that the diagnostic burden of 1-step testing is not justified by differences in pregnancy outcomes, increased diagnosis rates may still have long-term benefits for cardiometabolic health or offspring.
A diagnosis of gestational diabetes affects both individual patients and the health care system. The diagnosis can have life-changing effects, both positive and negative, on pregnant people, which may be particularly important when dietary recommendations conflict with cultural practices around food.39,40 Health system costs may increase, with a need for more endocrinologists, diabetes nurse educators or dietitians; additional sonograms; or more intensive monitoring during labour, delivery and the postpartum period. Conversely, increased diagnosis rates of gestational diabetes could have long-term public health cost savings or reduced morbidity from decreases in cardiometabolic diseases or metabolic effects on the offspring.41 Whether these benefits outweigh the burdens remains unclear.
Limitations
We cannot rule out the potential for unmeasured confounding by family history of diabetes, weight gain in early pregnancy or dietary or underlying genetic factors (possibly associated with race and ethnicity), as data were not available on these risk factors. We speculate that these confounders could account for the remaining 25% unexplained increase in gestational diabetes. However, we adjusted for place of birth of the pregnant person, which, although an imperfect indicator, did capture some of the potential confounding by race or ethnicity. Although pre-pregnancy BMI was missing in 26% of patients, our findings did not change substantially in stratified analyses and in analyses using imputation methods for missing data.
Conclusion
Our findings support the hypothesis that recent increases in the rate of gestational diabetes in BC were related to changes in screening practices rather than true changes in disease incidence. Although the generalizability of our findings to other jurisdictions is unknown, our study highlights the importance of having data on screening methods and completion to better understand the rising incidence of gestational diabetes observed elsewhere.
Footnotes
Competing interests: Michael Law has consulted for Health Canada and iTAD, and has provided expert witness testimony for the Federation of Post-Secondary Educators. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Elizabeth Nethery, Michael Law and Jennifer Hutcheon conceptualized and designed the study. Elizabeth Nethery conducted all analyses. All authors contributed to data interpretation. Elizabeth Nethery drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: Access to Population Data BC datasets provided by the data steward(s) is subject to approval but can be requested for research projects through the data steward(s) or their designated service providers. All inferences, opinions and conclusions drawn in this publication are those of the author(s), and do not reflect the opinions or policies of the data steward(s).
Funding: Elizabeth Nethery was supported by a Vanier Canada Graduate Scholarship. Michael Law holds a Canada Research Chair in Access to Medicines. Jennifer Hutcheon holds a Canada Research Chair in Perinatal Population Health. Funders had no role in this work.
- Accepted January 20, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/