Abstract
Background: With the declaration of the global pandemic, surgical slowdowns were instituted to conserve health care resources for anticipated surges in patients with COVID-19. The long-term implications on survival of these slowdowns for patients with cancer in Canada is unknown.
Methods: We constructed a microsimulation model based on real-world population data on cancer care from Ontario, Canada, from 2019 and 2020. Our model estimated wait times for cancer surgery over a 6-month period during the pandemic by simulating a slowdown in operating room capacity (60% operating room resources in month 1, 70% in month 2, 85% in months 3–6), as compared with simulated prepandemic conditions with 100% resources. We used incremental differences in simulated wait times to model survival using per-day hazard ratios for risk of death. Primary outcomes included life-years lost per patient and per cancer population. We conducted scenario analyses to evaluate alternative, hypothetical scenarios of different levels of surgical slowdowns on risk of death.
Results: The simulated model population comprised 22 799 patients waiting for cancer surgery before the pandemic and 20 177 patients during the pandemic. Mean wait time to surgery prepandemic was 25 days and during the pandemic was 32 days. Excess wait time led to 0.01–0.07 life-years lost per patient across cancer sites, translating to 843 (95% credible interval 646–950) life-years lost among patients with cancer in Ontario.
Interpretation: Pandemic-related slowdowns of cancer surgeries were projected to result in decreased long-term survival for many patients with cancer. Measures to preserve surgical resources and health care capacity for affected patients are critical to mitigate unintended consequences.
Declaration of the global COVID-19 pandemic led to the implementation of several clinical and policy-related measures to mitigate risk to vulnerable populations and conserve health care resources. Literature from early waves of the pandemic characterized patients with cancer as a vulnerable population.1,2 Moreover, cancer surgery can be highly resource intensive, which could strain the health care system’s ability to respond to the pandemic. Accordingly, in March 2020, the Ontario government recommended reducing the number of cancer surgeries, along with other elective surgeries performed in the province. These measures were aimed at reducing both patient morbidity and use of health care resources, primarily by decreasing routine postoperative admissions to wards and intensive care units, in anticipation of a potential surge of patients with COVID-19.3
Although necessary, this initial strategy resulted in a backlog of cancer surgeries, and some patients faced longer wait times to surgical treatment.4 Given clear evidence showing that longer surgical wait times can increase cancer-related risk of death, there is concern for the unintended consequences of the surgical slowdowns during the COVID-19 pandemic.5–8 International data have projected the negative impact on long-term survival associated with potential delays to cancer diagnosis or surgery across various cancer types.9–11 Recognizing the global differences in level of infection, response to the COVID-19 pandemic and cancer survival rates, country-specific data are required to understand local consequences and better guide future responses to times of resource constraint. As such, the objective of the current study was to evaluate the long-term implications of pandemic–related cancer surgery slowdowns on cancer survival in Ontario, Canada.
Methods
Study design
We used an individual-level, discrete time, health state–transition model to forecast survival outcomes with pandemic-related cancer surgery slowdowns. This study focused on patients with cancer receiving nonemergent cancer surgery in Ontario. Our study included patients with breast, gastrointestinal, genitourinary, gynecological, head and neck, hepatobiliary, lung and prostate cancers. We used this model to evaluate survival outcomes among modelled populations of patients with cancer who underwent surgery in resource settings before the pandemic, compared with during the pandemic.
State-transition model
We simulated a model population of patients awaiting cancer surgery over a 6-month period in Ontario, Canada. This population included patients with a decision to proceed to cancer surgery who were on the wait-list at the beginning (day 1) of the pandemic, as well as new referrals for cancer surgery that we simulated as additional patients entering the microsimulation on weekdays over a 6-month time period. The model population was stratified by cancer disease site.
Patients already on the wait-list on day 1 were assigned an initial wait time. Upon model entry, these individuals could proceed with cancer surgery if an operating room was available. If no operating room was available, simulated patients would remain in the model’s wait-list health state. All simulated incident patients who entered the model after day 1 were placed on the wait-list.
We ran the model with daily cycles (i.e., daily time steps) such that, each day, patients could proceed to the operating room if resources were available. If an operating room was unavailable, patients would enter or remain on the wait-list where they would accrue time. For patients on the wait-list, additional time was added for weekends (assuming no operating room would be available on Saturday or Sunday) and for patient-related delays (e.g., preoperative medical optimization, travel time). From this, the model calculated the mean duration on the wait-list, stratified by cancer disease site.
We modelled long-term survival over a 10-year time horizon. For those patients that did not have any pandemic-related increase in wait time, survival was modelled based upon historical survival data from patients who underwent cancer surgery in Ontario. To model the survival implications from longer wait times to cancer surgery, we derived the difference in mean wait time between the simulated prepandemic population and simulated pandemic scenarios, stratified by disease site. The difference in the simulated mean wait time was then used to estimate the risk of death owing to an incremental increase in wait time by applying literature-derived hazard ratios (HRs) for risk of death per day to the difference. The model was implemented using TreeAge Healthcare Pro 2021 software (TreeAge Software Inc.). Details on data input and model calibration can be found in Appendix 1, Supplemental File 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202380/tab-related-content.
Base-case analysis
We conducted a base-case analysis to evaluate the survival outcomes for patients with cancer in Ontario who faced pandemic-related increases in wait times for curative-intent surgery owing to surgical slowdowns. In our model, pandemic operating room resources were simulated to reflect the observed Ontario response to the global pandemic. Real-world data on surgical activity during the pandemic were used to inform our model’s simulated pandemic resources such that simulated operating room resources were at 60% for the first month of the pandemic, 70% for the second month and 85% for months 3–6 (Appendix 1, Supplemental Figure 1). Volumes of simulated patients, by disease site, were based on real-world estimates of surgical volumes in 2019. Using Ontario data to provide estimates, we excluded simulated patients who may have received systemic therapy or radiation therapy as a mitigation strategy to manage expected increases in wait times from the pandemic analysis (Appendix 1, Supplemental Table 2). As a comparator, we also simulated a prepandemic population of patients awaiting cancer surgery with 100% available operating room resources. Additional methodological details can be found in Appendix 1, Supplemental File 1. The primary outcomes included life-years lost per patient and life-years lost among the affected Ontario population. We also included a secondary outcome of 10-year survival for the affected Ontario cancer population.
Scenario analysis
We conducted scenario analyses to explore the long-term implications of hypothetical scenarios of more restricted access to cancer surgery early in the pandemic. Specifically, these scenarios allowed for characterization of the relative change in life-years lost in hypothetical scenarios of more restricted access to cancer surgery. These pandemic surgical slowdown (PSS) scenarios included one in which operating room resources for cancer surgery were maintained at 60% for the first 6 months (PSS-1), as well as an alternative scenario that evaluated the implications of a 2-month slowdown at 60% operating room resources, followed by 75% for months 3–6 (PSS-2).
To characterize the uncertainty in the HRs used to estimate risk of death with longer wait times to cancer surgery, we conducted wait time mortality (WM) scenario analyses by varying the used per-day HR to 1 of 3 scenarios: lower risk of death owing to longer wait times for all cancers (WM-1), higher risk of death owing to longer wait times for all cancers (WM-2) and higher risk of death owing to longer wait times for cancers with high risk of progression (WM-3).
Ethics approval
Ontario Health (Cancer Care Ontario) and ICES are designated as “prescribed entities” for the purposes of section 45(1) of the Personal Health Information Protection Act of 2004. As such, they are authorized to collect personal health information from health information custodians without patient consent, and to use this information for the purpose of analysis or compiling statistical information with respect to the management, evaluation or monitoring of the allocation of resources to or planning for all parts of the health system, including the delivery of services. Projects that use data collected by ICES under section 45 of the Act, and use no other data, are exempt from ethics board review. The use of the data in this project is authorized under section 45 and approved by ICES’ Privacy and Legal Office.
Results
Based on real-world data from 2019, we estimated that 4639 patients were already on the wait-list for surgery on the first day of the pandemic, ranging from 158 patients with hepatobiliary cancers to 1619 patients with genitourinary cancers, and that an additional 140 new patients would be added to the wait-list every day (Table 1).
Data inputs for the microsimulation models
Base-case analysis
Table 2 summarizes the results of the base-case analysis of the prepandemic and pandemic populations. The simulated prepandemic model population comprised a total of 22 799 patients awaiting cancer surgery over a 6-month period between March 2019 and September 2019. The mean time on the wait-list for this modelled population, in the setting of simulated normal operating room resources, was 25 days. The simulated pandemic model population comprised 20 177 patients, after exclusion of patients who received alternative treatments as a mitigation strategy to manage potential delays to cancer surgery. The mean wait time was 32 days after an initial slowdown to 60% operating room resources for month 1, 70% for month 2 and 85% for months 3–6, compared with a mean wait time of 25 days before the pandemic (incremental increase 7 d). This incremental increase in wait time resulted in an expected 843 (95% credible interval [CrI] 646–950) life-years lost among patients with cancer in Ontario (Table 2).
Results from the base-case analysis for wait time, life-years lost and 10-year survival in the prepandemic and pandemic simulation models*
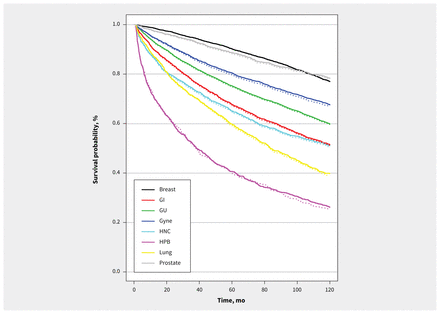
The largest changes in life-years lost per patient were seen for patients with genitourinary (0.07 life-years lost, 95% CrI 0.06–0.07), gastrointestinal (0.05 life-years lost, 95% CrI 0.05–0.06) and head and neck (0.05 life-years lost, 95% CrI 0.03–0.06) cancers. Survival at 10 years decreased by 0.3%–0.9% across cancer disease sites in the pandemic model compared with the prepandemic model, with the greatest change observed in patients with hepatobiliary cancers (26.0% prepandemic v. 25.1% pandemic) (Table 2 and Figure 1).
Modelled survival outcomes from base-case analysis of simulated prepandemic (solid line) and pandemic (dotted line) populations, by cancer disease site. Note: GI = gastrointestinal, GU = genitourinary, Gyne = gynecological, HNC = head and neck, HPB = hepatobiliary.
Pandemic scenarios
With a hypothetical 60% reduction in operating room resources for cancer surgery for the first 6 months of the pandemic (PSS-1), incremental increases in wait time of 10–21 days, compared with prepandemic wait times, translated to 0.01–0.11 life-years lost per patient and reductions in 10-year survival by 0.3–1.6 percentage points, across cancer sites (Table 3). Overall, this translates to 1539 life-years lost among the population of Ontario patients requiring cancer surgery.
Results of pandemic surgical slowdown scenario analyses modelling alternative, hypothetical reductions in operating room resources
In the less restrictive scenario in which operating room resources for cancer surgery were reduced to 60% for the first 2 months and 75% for the next 4 months of the pandemic (PSS-2), wait times were shorter than in PSS-1 (incremental increase 8–19 d), translating to fewer life-years lost among the cancer population (1306 life-years lost).
Scenarios characterizing uncertainty in risk of death
Among patients with breast, gastrointestinal, genitourinary, gynecological and prostate cancers, variation in the HRs for risk of death, associated with longer wait times, led to minimal deviations in expected life-years lost per patient (Appendix 1, Supplemental Table 4). The largest variation in life-years lost per patient was seen among cancers with a higher risk of progression (i.e., hepatobiliary and head and neck cancers), with a more than 1.5-fold increase in life-years lost with increases in the HR for risk of death associated with longer wait times.
Interpretation
In modelling of the long-term survival outcomes of COVID-19 pandemic-related cancer surgery slowdowns in Ontario, we showed variation in loss of life-years across cancer disease sites and notable increases in life-years lost if slowdowns were more restrictive. The health care response to the COVID-19 pandemic in Ontario was driven by an intention to protect vulnerable populations of patients and reserve adequate health care resources to manage a potential surge of patients with COVID-19. We have shown the likely unintended consequences of this policy intervention in Ontario. These results highlight the importance of data-driven strategies to prioritize cancer surgery during times of surgical resource constraint to mitigate these long-term consequences for patients with cancer.
As expected, we observed differential effects of risk of death based on cancer disease site, with the largest life-years lost seen among those cancers known to be at higher risk of death. Although our model was a simplification of the diverse disease trajectories, the notable differences in survival by disease site suggest a need for measures of surgical prioritization during pandemic-related slowdowns.
Soon after the onset of the pandemic, Ontario Health – Cancer Care Ontario developed guidance documents to inform surgical prioritization during surgical slowdown; however, most patients with cancer were prioritized into a moderate risk category. 12 Prioritization should not occur only within services, but rather across surgical services and cancer sites. Our data, which showed differential long-term risk of death for different cancers, can be used to refine surgical prioritization in future settings of limited surgical resources. Nonetheless, balancing poorer oncologic outcomes in patients with cancer with the overall goals of a health care system need to be considered, and future models should incorporate noncancer surgeries, as well as account for the system’s ability to manage a surge from a human and physical resource perspective.
Among accumulating data on the unintended consequences of the COVID-19 pandemic for patients awaiting cancer surgery, our data characterized the impact of pandemic-related surgical slowdowns for patients with cancer in Ontario.9–11 In contrast to previous literature, our results characterized the survival implications of longer wait times based on actual data of surgical volume slowdowns from the initial response to the pandemic in Ontario. Although our results are in keeping with the previous literature, the expected life-years lost from our data are lower than other studies, owing to the shorter simulated increases in wait times in our model and to conservative model estimates.10,11 Thus, this model is felt to better represent observed surgical slowdowns during the initial pandemic response more than assumed or hypothetical delays to cancer surgery. Nevertheless, the results of our pandemic scenario analysis — which showed a larger, deleterious impact on long-term survival with more restrictive access to cancer surgery — resonate with previous literature and highlight the importance of prioritizing cancer surgeries during times of surgical resource constraint. The absolute values of losses in life-years across these restrictive scenarios can be viewed as offsetting some of the gains that have been achieved through advances in oncology care.13–17 Similar to how time, resources and finances have been invested to support these advances in oncology, the same investment should be directed toward strategies to avoid future impacts on timely access to cancer surgery.
During the pandemic, the volume of incident patients with cancer dropped, compared with prepandemic periods, thereby leading to a cohort of “missing” patients. This is likely attributed to diagnostic delays with fewer patients proceeding through routine cancer screening or other diagnostic pathways.18 These diagnostic delays pose the risk of stage migration, with early data describing a reduction in the reported incidence of early stage cancers with a concurrent increase in stage IV diagnoses among cancers with established screening programs.19 To account for these potential missing patients, our model simulated patient volumes based on prepandemic cancer surgery volumes. However, given a lack of comprehensive, cancer-specific data, we did not incorporate diagnostic delays and stage migration into this model. Recognizing the substantial differences in survival outcomes associated with advanced (as opposed to early-stage) cancer there are likely to be additional life-years lost because of delayed presentation of the missing patients during the pandemic. As such, our results are likely a conservative estimate of the true impact of the COVID-19 pandemic on outcomes of patients with cancer.
Limitations
All data used in this model are specific to patients with cancer treated in Ontario for the specific cancer types included, and this may limit generalizability. Interjurisdictional variability in surgical slowdowns (for instance, across the provinces) would translate to interjurisdictional differences in patient outcomes. Our model considered the potential use of additional therapies that may be offered before surgery (e.g., systemic therapy, radiation therapy) to mitigate the effects of increasing wait times to treatment by removing these patients from our simulated wait-list under the assumption that the survival of these patients would be similar to those who underwent surgery without delay. However, we acknowledge there may be survival implications of these mitigation strategies that could not be incorporated because of a lack of evidence of the clinical efficacy of these strategies. In addition, as it was expected that inclusion of patients who underwent mitigation strategies would likely have been associated with an increase in wait time and resultant decrease in expected long-term survival, we reinforce that our estimates for life-years lost are likely conservative. Finally, the HRs used to model the effect of longer wait times on risk of death were not cancer-specific and thus, may either over- or under-represent outcomes across specific cancer disease sites. However, our approach to incorporate previously used per-day HRs for cancers with similar 5-year survival rates has been used by other international modelling studies and allows for comparability.
Conclusion
Longer wait times from slowdowns of cancer surgeries during the COVID-19 pandemic are projected to lead to decreased long-term survival for many patients with cancer. Future research should characterize the additional impact of pandemic-related diagnostic delays and stage migration on patient outcomes. Although de-escalation of cancer surgeries during the pandemic may be required to protect vulnerable populations and create health care capacity, these slowdowns are associated with a risk of unintended harm. Careful management of health care resources is critical during times of resource constraint to mitigate unintended consequences.
Acknowledgements
The authors thank Ravleen Vasdev for administrative support with this manuscript, and Louis Everest, Qinq Li (ICES) and Jonathan Wang (Ontario Health–Cancer Care Ontario) for their assistance with data collection.
Footnotes
Competing interests: Beate Sander is co-chair of the Ontario COVID-19 Modeling Consensus Table and reports funding from the Canadian Institutes of Health Research, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All authors contributed to the design of the study. Ambica Parmar, Antoine Eskander and Jonathan Irish collected data. Ambica Parmar, David Naimark and Kelvin Chan contributed to data analysis and all authors contributed to interpretation. Ambica Parmar drafted the manuscript. All authors critically revised it, gave approval for final publication and agreed to be accountable for all aspects of the work.
Funding: This work is funded by a Sunnybrook Research Institute and Sunnybrook Foundation COVID-19 Response Grant.
Data sharing: Data used in this study include data from ICES. This data set is held securely in coded form at ICES. Although data-sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors on request, with the understanding that the computer programs may rely on coding templates or macros that are unique to ICES and are therefore inaccessible or may require modification. Additional data included in this study are from Ontario Health (Cancer Care Ontario). As the original source data are not owned by the authors, they cannot be made publicly available.
Disclaimer: This study was supported by the Canadian Centre for Applied Research in Cancer Control, which is funded by the Canadian Cancer Society (grant no. 2015-703549). This study was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information provided by Ontario Health (Cancer Care Ontario; OHCCO), the Ontario Cancer Registry (OCR) and the Canadian Institute for Health Information (CIHI), and information on deaths from Service Ontario’s Office of the Registrar General. The analyses, conclusions, opinions, and statements reported in this article are those of the authors and do not necessarily reflect those of these organizations or the funding sources. No endorsement by the ICES, Ontario Health, OH-CCO, Service Ontario, MOH, MLTC or CIHI is intended or should be inferred.
- Accepted January 10, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/