Headaches, palpitations, and diaphoresis are the classic triad of pheochromocytoma, but not all patients with pheochromocytoma are symptomatic.
Pheochromocytoma crisis can occur spontaneously or be precipitated by medications, including β-adrenergic receptor blockers and sympathomimetics.
Pheochromocytomas may lead to Takotsubo and reverse Takotsubo cardiomyopathies.
Pheochromocytoma-induced hypertension should be treated with α-adrenergic blockade before β-adrenergic blockade to prevent severe hypertensive episodes.
A 66-year-old woman presented to the emergency department with a 1-week history of productive cough and malaise. She had a history of type 2 diabetes and hypothyroidism, and no history of hypertension. Her medications were metformin, gliclazide, and levothyroxine. Two days before presentation, her family doctor had prescribed oral amoxicillin for a respiratory tract infection, and she had started taking an over-the-counter nasal decongestant spray containing pseudoephedrine. She described an unexplained 20-pound weight loss over the last year and intermittent palpitations. She did not smoke and used alcohol infrequently. Her mother had passed away from colon cancer and her half sister had lung cancer; she reported no endocrinopathies in first-degree relatives.
The patient’s heart rate was 150 beats per minute, blood pressure 144/99 mm Hg, and oxygen saturation above 95% on room air. She was afebrile. She appeared diaphoretic, hypovolemic (dry mucous membranes, low jugular venous pressure), and had no signs of respiratory distress such as tachypnea or accessory respiratory muscle use. Laboratory investigations showed a leukocyte count of 14.0 (normal range 4.0–11.0) × 109/L, with neutrophilia of 12.4 (normal range 2.0–7.5) × 109/L. Her creatinine level was 110 μmol/L; the only previous result available was 70 μmol/L 4 years earlier, and lactate was 2.4 (normal range 0.5– 2.2) mmol/L. Her initial high-sensitivity troponin level was 32 (normal ≤ 16 ng/L) and the result of a repeat test 4 hours later was unchanged. Her glucose level was elevated, at 19.3 (normal range 3.6–7.7) mmol/L, and thyroid-stimulating hormone level was 2.9 (normal range 0.35–4.94) mIU/L. An electrocardiogram showed sinus tachycardia. We ordered a computed tomography (CT) pulmonary angiogram to assess for pulmonary embolism, which showed right upper and left lower lobe ground-glass opacities, suggesting community-acquired pneumonia with no findings of pulmonary embolism. A mass in the left upper abdomen was also seen.
Initially, we ordered 1 g of ceftriaxone intravenously (IV) to treat community-acquired pneumonia and infused 3 L of Ringer’s lactate IV over 5 hours. We managed the patient’s hyperglycemia with sliding-scale insulin and requested a CT scan of her abdomen to characterize the mass.
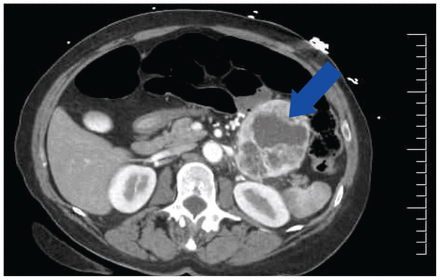
The next morning, about 15 hours after the patient’s initial presentation, her hypovolemia had resolved (jugular venous pressure, lactate, and creatinine levels had normalized) but her tachycardia persisted. We ordered an echocardiogram, which showed normal biventricular function. That night, the patient’s heart rate remained elevated at 140 beats per minute, and her systolic blood pressure rose to 150 mm Hg. In consultation with the cardiology team, the on-call physician ordered 25 mg of oral metoprolol to treat palpitations and hypertension, as well as the ongoing sinus tachycardia. Her heart rate decreased to 120 beats per minute, but blood pressure remained elevated. The abdominal CT that evening showed an 8-cm left retroperitoneal mass with cystic and solid features, possibly adrenal in origin, without extra-adrenal disease (Figure 1). We requested 24-hour urine cortisol, plasma renin and aldosterone levels, and serum and urine metanephrines.
Computed tomography scan with contrast of the abdomen of a 66-year-old woman with a possible adrenal mass in the left upper quadrant, concerning for pheochromocytoma (blue arrow).
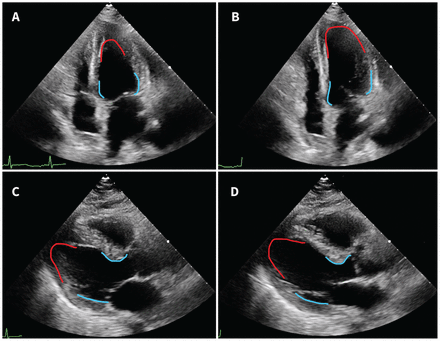
Five hours after receiving metoprolol, the patient’s blood pressure increased to 173/125 mm Hg, she developed tachypnea, and her oxygen requirements increased. Her troponin level increased to 10 160 ng/L and her electrocardiogram showed new anterior ST depression. A repeat echocardiogram revealed a hyperdynamic apex with global akinesis, concerning for reverse Takotsubo cardiomyopathy (TCM) (Figure 2; 2 supplementary videos are available at Appendix 1, at www.cmaj.ca/lookup/doi/10.1503/cmaj.231575/tab-related-content).
Echocardiogram showing akinesis of basal segments (blue markings) and a hyperdynamic apex (red markings) suggesting reverse Takotsubo cardiomyopathy. Images include apical 4-chamber view in end-systole (A) and end-diastole (B), as well as parasternal long axis view in end-systole (C) and end-diastole (D).
We transferred the patient to the intensive care unit (ICU) and inserted an endotracheal tube. Three hours after intubation, she developed severe hypotension, with systolic blood pressure of 70 mm Hg. She developed cardiogenic shock and, despite multiple vasopressors, required veno-arterial extracorporeal membrane oxygenation (ECMO). Cardiac CT showed no coronary artery atherosclerosis.
The results of the patient’s endocrine investigations showed elevated levels of metanephrines of 221.13 (normal 0–0.50) nmol/L, normetanephrines of 121.71 (normal 0–0.90) nmol/L, and 24-hour urine metanephrines of 4053 (normal 0–170) nmol/d, confirming a diagnosis of pheochromocytoma. Her 24-hour urine cortisol and plasma renin-aldosterone ratio were normal.
The patient’s hemodynamic instability improved and ECMO was discontinued 3 days after it was started. In the ICU, physicians prescribed doxazosin at a dose of 2 mg/d, titrated up by 2 mg increments per day to 16 mg/d. Four days after initiating doxazosin, we started the patient on metoprolol to treat persistent tachycardia, at a dose of 12.5 mg twice daily, and titrated to 25 mg twice daily as her blood pressure tolerated. On these medications, her average blood pressure was less than 130/80 mm Hg, heart rate less than 80 beats per minute, and we discharged her home. To expand plasma volume, we advised liberal fluid intake (2–3 L/d) and salt intake (5–10 g/d).
Forty-six days after discharge, the patient underwent complete surgical removal of the adrenal mass. Pathology results confirmed the diagnosis of pheochromocytoma. Her immediate postoperative blood pressure was 99/62 mm Hg, and a few weeks later, it was 99/57 mm Hg without medications. Her postoperative plasma metanephrines level was 0.07. Genetic screening for familial disorders associated with pheochromocytoma — including succinate dehydrogenase disorders, neurofibromatosis 1, von Hippel–Lindau syndrome, and multiple endocrine dysplasia 2 — was negative.
Discussion
Pheochromocytoma is a rare tumour arising from chromaffin cells in the adrenal medulla, leading to uncontrolled catecholamine synthesis and secretion. Pheochromocytomas are the underlying cause of hypertension in 0.2%–0.4% of patients, with 2–8 cases detected annually per million people.1 Headaches, palpitations, and diaphoresis are the classic triad of pheochromocytoma, but patients may experience other signs and symptoms including nausea, vomiting, tremors, anxiety, and chest pain (Table 1).2 Hypertension can be paroxysmal or sustained. About 5%–8% of patients with pheochromocytoma are asymptomatic. Asymptomatic pheochromocytoma is usually associated with large cystic tumours or familial syndromes including von Hippel–Lindau syndrome, multiple endocrine dysplasia 2, succinate dehydrogenase disorders, and neurofibromatosis 1.1,3 Because the symptoms associated with hypersecretion of catecholamines can mimic several other medical conditions, time until diagnosis may be delayed. Early recognition and diagnosis is key before the development of severe, and in some cases fatal, cardiovascular complications. About 10%–17% of adrenal pheochromocytomas are malignant.4
Signs and symptoms of pheochromocytoma and pooled sensitivities2
Diagnosing pheochromocytoma requires biochemical confirmation of excessive catecholamine release and imaging to localize the tumour. Elevated plasma-free metanephrines and 24-hour urine fractionated metanephrines have the highest sensitivity, of 98% and 93%, respectively, with a specificity of 94% for both.5 In adults, CT is the preferred imaging modality for diagnosis because its spatial resolution is superior to that of magnetic resonance imaging.4 Functional imaging studies such as meta-iodobenzylguanidine (MIBG) or positron emission tomography–CT scans may also be used.
Hypertension and tachycardia in patients with pheochromocytoma must be managed before surgery. α-Adrenergic blockers should be prescribed first and then dose titrated until blood pressure is no longer elevated. β-Blockers should be started only after successful α-adrenergic blockade, to mitigate the risk of life-threatening hypertension and cardiopulmonary decompensation from unopposed α-adrenergic stimulation. α-Adrenergic blockade can be achieved with the nonselective agent phenoxybenzamine or selective agents such as prazosin, terazosin, or doxazosin. Phenoxybenzamine, an irreversible α-blocker, is more effective in preventing intraoperative hemodynamic instability but is not readily available.6 According to retrospective data, a target blood pressure of less than 130/80 mm Hg while seated and greater than 90 mm Hg systolic while standing, with a target heart rate of 60–70 beats per minute seated and 70–80 beats per minute standing, is desired before surgical resection of the tumour, usually 7–14 days after medical optimization.4
A life-threatening complication of pheochromocytoma is a pheochromocytoma crisis, which can lead to hemodynamic instability and end-organ dysfunction. It can occur spontaneously, due to trauma, or be triggered by medications, including β-adrenergic receptor blockers, sympathomimetics (e.g., pseudoephedrine, ephedrine), dopamine receptor antagonists, opioid analgesics, neurotransmitter reuptake blockers, and peptide and steroid hormones (e.g., glucagon, adrenocorticotrophic hormone, corticosteroids). 4,7 The clinical presentation of a pheochromocytoma crisis ranges from severe hypertension to circulatory failure and shock.
Pheochromocytoma has uncommonly been associated with TCM or reverse TCM.8 Takotsubo cardiomyopathy, popularly known as “broken heart syndrome,” is characterized by transient regional systolic dysfunction with normal coronary oxygen supply, usually in the setting of extreme emotional or physical stress associated with high endogenous catecholamine activity. Typical TCM is characterized by transient hypokinesis of the left ventricular apex. Generally, it affects females older than 55 years and resolves within a week of presentation. Although the pathogenesis is not well understood, the association with physical or emotional stress suggests it may be caused by catecholamine-induced coronary microvascular vasoconstriction or spasm leading to myocardial stunning.9 Other potential causes of TCM include myocarditis or direct catecholamine-associated myocardial toxicity.9 Reverse TCM is a rare variant of TCM, featuring apical hyperkinesis and basal or inferior wall hypokinesis. Case studies have reported that ECMO can be used to successfully manage pheochromocytoma-induced TCM or cardiogenic shock, as shown in our patient.10
This patient experienced an uncommon occurrence of reverse TCM triggered by a pheochromocytoma crisis, which was likely provoked by the administration of metoprolol before adequate α-blockade. Additionally, the patient’s use of a nasal decongestant spray containing pseudoephredine before presentation likely exacerbated her initial tachycardia. The absence of hypertension and classic symptoms of pheochromocytoma made the diagnosis especially challenging.
Two videos showing transthoracic echocardiogram views of reverse Takotsubo cardiomyopathy in a 66-year-old patient are available in Appendix 1 (at www.cmaj.ca/lookup/doi/10.1503/cmaj.231575/tab-related-content). Video 1 shows the apical 4-chamber view and video 2 shows the parasternal long axis view.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca
Footnotes
Author video summary at www.cmaj.ca/lookup/doi/10.1503/cmaj.231575/tab-related-content.
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/