Through genomic sequencing, some patients with previously undiagnosed autoinflammatory diseases are now understood to have a new syndrome called vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome.
VEXAS syndrome is caused by somatic mutations in the UBA1 gene, and sequencing of this gene is the definitive diagnostic test.
Features for consideration of VEXAS syndrome in patients with autoinflammatory symptoms include dermatologic manifestations, myelodysplastic syndrome or monoclonal gammopathy, and vacuolation of myeloid and erythroid precursors in bone marrow.
Advances in genomic medicine will continue to uncover causes of previously undiagnosed diseases.
Throughout the history of medicine, technological progress has enabled physicians to understand and diagnose previously enigmatic syndromes. Although Hippocrates described many of the features of tuberculosis, it was not until the invention of the microscope that physicians understood the cause to be Mycobacterium tuberculosis. In a similar way, the technology and application of genome sequencing (genomics) is now revolutionizing the understanding of many diseases and syndromes.1
In 2001, the first effort to sequence the human whole genome took more than 5 years; it is now possible to generate clinically actionable results from whole genome sequencing in as little as 5 days.2 The wealth of data generated by these techniques has shown that many undiagnosed syndromes have specific genetic causes.
We illustrate the power of genomics to affect clinical diagnostics and practice with the newly discovered vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome, which has provided a unifying genetic diagnosis for a group of patients with a previously undiagnosed autoinflammatory syndrome. This syndrome is relevant to many physicians as it can present with a wide variety of symptoms involving many organ systems and it has an adult age of onset, which shows that genetically driven disorders can affect almost any patient. At present, many patients with VEXAS are placed within existing diagnostic categories and will benefit from accurate diagnosis based on the genomic driver of their disease.
What are autoinflammatory syndromes and how can they present?
Autoinflammatory syndromes (also known as systemic auto-inflammatory diseases) are defined by the presence of pathogenic hyperactivation of immune pathways in an antigen-independent manner. More than 40 distinct monogenic conditions that can lead to an autoinflammatory syndrome have been described.3 The exact features depend on the specific disease, but a common presentation is fever of unknown origin, defined as a persistent fever (> 38.3°C for > 3 wk) without an obvious cause after appropriate initial investigations. In a recent prospective study from the Netherlands of 73 patients with fever of unknown origin, an autoinflammatory syndrome was identified in 22% of patients.4 Autoinflammatory syndromes should be considered for patients with fever of unknown origin for whom extensive investigation for infection, malignant disease, hemophagocytic syndrome and other causes of fever and systemic inflammation have been excluded.
What is VEXAS syndrome and when should it be considered as a diagnosis?
Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome was first described in December 2020 after whole genome sequencing was performed for a cohort of patients as part of the Undiagnosed Diseases Program at the National Institutes of Health (NIH).5 By integrating large-scale gene mutation data with information on patients’ symptoms, the NIH identified 25 individuals with similar syndromic features who all had mutations in the X-linked UBA1 gene, which produces an enzyme that is important in recycling proteins within cells.5 This mutation was confirmed to be somatic (i.e., acquired and not inherited), and was shown to reproduce some features of VEXAS when inserted into an animal model.5 Shortly after this landmark publication, the first case of VEXAS in Canada was identified.6
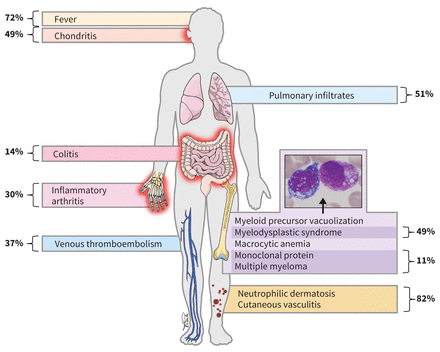
VEXAS is an adult-onset autoinflammatory syndrome with several unique clinical and pathologic features that distinguish it from other known rheumatologic diseases.5 Patients with VEXAS present in late adulthood (median age 71 yr) with symptoms that include recurrent high-grade fevers, dermatologic manifestations (e.g., neutrophilic dermatoses, cutaneous vasculitis), chondritis of the ear and nose, pulmonary infiltrates and cytopenias (Figure 1). Although these are the most common manifestations, recent reports have suggested additional associated symptoms, including uveitis, gastrointestinal pain or inflammation, aortitis and hepatosplenomegaly.7,10 Many patients with VEXAS syndrome have previously received a diagnosis of relapsing polychondritis, Sweet syndrome or polyarteritis nodosa. Since VEXAS is an X-linked disease, most patients are male, but female patients have been reported.13 Patients often have severe and progressive symptoms that eventually result in end-organ compromise; about 28% of patients described in a recent series died of their disease (Table 1).5,7
The clinical findings in patients with vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome often include high-grade fevers, dermatologic manifestations (e.g., neutrophilic dermatoses, cutaneous vasculitis), chondritis, pulmonary infiltrates, macrocytic anemia, myelodysplastic syndrome or monoclonal proteinemia, and steroid dependence. The characteristic vacuolation of myeloid precursors is shown in the pull-out panel. The frequency of each manifestation is noted in bold.5,7–12
Features of vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome from published cohorts*
VEXAS also results in characteristic hematologic manifestations. Almost all patients with this syndrome have macrocytic anemia, and many have a concomitant low-grade myelodys-plastic syndrome, monoclonal proteinemia or multiple myeloma. Patients with VEXAS syndrome have morphological features in their bone marrow that are highly specific to the diagnosis, notably vacuolation of myeloid and erythroid precursors. These features may not be described on pathology reports, and a pathologist should specifically look for these findings in patients suspected of having VEXAS.
Given the recency of its discovery, the incidence and prevalence of VEXAS syndrome are unknown, although we expect it will be identified more frequently once knowledge of the diagnosis and the testing availability is more widespread.
How can a diagnosis of VEXAS syndrome be confirmed?
The diagnostic work-up for a patient with suspected VEXAS syndrome should include investigations of inflammatory markers (e.g., C-reactive protein, serum ferritin), a bone marrow biopsy, pulmonary imaging, pulmonary function tests and the necessary investigations to rule out alternative diagnoses. However, VEXAS syndrome can be confirmed only by the presence of a somatic UBA1 mutation, usually in peripheral blood. At present, access to testing for this mutation is variable, given how recently the diagnosis was described. The availability of testing should be discussed with local experts who have seen patients with VEXAS syndrome or who can help arrange sequencing of the UBA1 gene. Contacting local hematologists or rheumatologists with an interest in auto-inflammatory syndromes or myelodysplastic syndrome is advised. In the future, a coordinated Canadian network of providers with expertise in VEXAS syndrome and related disorders may be useful.
How are patients with VEXAS syndrome treated and by whom?
Patients with VEXAS syndrome are typically dependent on steroids, and corticosteroids are usually the only therapy that transiently improves their inflammatory symptoms. Given that many patients will have been treated with steroids for several years, complications caused by long-term steroid use are common, and should be considered when assessing treatment modalities for VEXAS syndrome (e.g., allogeneic hematopoietic stem cell transplant). Steroid-sparing agents or disease-modifying antirheumatic drugs are ineffective in most patients with VEXAS syndrome, and therapies directed at the malignant myeloid clone are likely the most effective approach. In a recent study, about 50% of patients with VEXAS syndrome and concomitant myelodysplastic syndrome who had highly refractory inflammatory symptoms improved after treatment with a hypomethylating agent, which is a standard therapy for de novo myelodysplastic syndrome.14 The only therapy likely to have long-term curative potential is allogeneic hematopoietic stem cell transplantation. Although this comes with serious risks, transplantation has been successful in several patients15 and a phase 2 trial is underway (ClinicalTrials.gov registration No. NCT05027945). Knowledge regarding the effectiveness of treatments for patients with VEXAS syndrome will likely evolve rapidly in the near future.
Patients with VEXAS syndrome should be managed with a multidisciplinary approach, typically with involvement from rheumatologists, hematologists and infectious disease specialists. Eligibility for allogeneic hematopoietic stem cell transplantation should be determined early in the disease course, as VEXAS syndrome-related complications (e.g., advanced pulmonary fibrosis, persistent infections) can eventually limit this as a treatment option.
How might genomic medicine influence clinical practice in the future?
The discovery of VEXAS syndrome is an example of how genomics will redefine many aspects of the practice of medicine in the future. In some fields, such as hematology and oncology, the use of gene sequencing panels has become routine and standard of care for many patients. As more is understood about the contributions of genetic alterations to human disease, genomic medicine will be increasingly incorporated into clinical practice.
CMAJ invites contributions to Innovations, which highlights recent diagnostic and therapeutic advances. Novel uses of older treatments will also be considered. For publication, the benefits of the innovation, its availability and its limitations must be highlighted clearly, but briefly. Visual elements (images) are essential. Submit brief evidence-based articles (maximum 1000 words and five references) to http://mc.manuscriptcentral.com/cmaj or email andreas.laupacis{at}cmaj.ca to discuss ideas.
Footnotes
Competing interests: Luke Chen reports speaker fees from GlaxoSmithKline, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributions: All authors contributed to the conception and design of the work, drafted the manuscript, revised it critically, gave approval for the final version to be published and agreed to be accountable for all aspects of the work.
Funding: Ryan Stubbins is supported by grants from the Leukemia Lymphoma Society of Canada, the Canadian Institutes of Health Research (202002LFC-439884) and the Clinician Investigator Program at the University of British Columbia.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/