Heterologous vaccination with BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines may be associated with higher rates of vaccine-related myopericarditis in males.
Myopericarditis related to SARS-CoV-2 mRNA vaccine may uncommonly persist for months, with ongoing troponin elevation and abnormalities on cardiac magnetic resonance imaging.
Nonsteroidal anti-inflammatory drugs and colchicine therapies are effective in treating the symptoms of myopericarditis related to SARS-CoV-2 mRNA vaccines.
A healthy 41-year-old man on vacation presented to the emergency department of a local hospital with central, constant, stabbing chest discomfort that worsened with inspiration. He reported no recent illness, and no medication or recreational drug use. His only medical history was Gilbert syndrome. He had received a BNT162b2 SARS-CoV-2 vaccine 4 months previously and an mRNA-1273 SARS-CoV-2 vaccine 2 weeks previously. His clinical examination was unremarkable. However, a high-sensitivity troponin I level was elevated (1200 [normal < 45] ng/L), and an electrocardiogram showed diffuse ST elevation. Nasopharyngeal polymerase chain reaction testing for SARS-CoV-2 was negative. Given suspicion of acute myocardial infarction, he underwent a coronary angiogram, which was normal. A transthoracic echocardiogram showed preserved cardiac function and no pericardial effusion. The patient was discharged after a 3-day admission with a diagnosis of mRNA vaccine–related myopericarditis, and arrangements were made for outpatient follow-up with a cardiologist. He was free of pain at discharge, and no outpatient medications were prescribed.
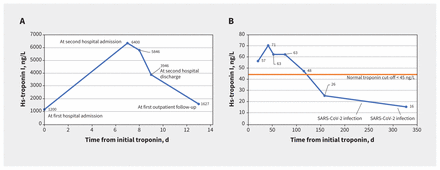
Four days after discharge, after returning home, the patient’s chest pain worsened, and he presented to the emergency department of our hospital with intense discomfort. He rated the pain at a 10 out of 10, and described it as left-sided, nonradiating, stabbing, worse with inspiration and not improved with rest. His blood pressure was 121/83 mm Hg; his heart rate was 75 beats/min and regular, with a normal cardiovascular examination. He had no rales on auscultation. His chest radiograph was normal. An electrocardiogram showed sinus rhythm, no conduction abnormalities, borderline inferior T wave changes and no ST deviation (Figure 1). Blood work showed elevated high-sensitivity troponin I (6400 ng/L; normal < 20 ng/L for Beckman Coulter assay), a brain natriuretic peptide level of 30 (normal < 100) ng/L and a C-reactive protein level of 3.7 (normal < 8.0) mg/L (Figure 2A). His serum creatinine was normal.
Electrocardiogram of a 41-year-old man on presentation to our hospital, showing sinus rhythm with nonspecific infero-apical T wave abnormalities and no ST segment deviation.
Temporal trend in high-sensitivity (hs) troponin I in (A) weeks 1–2 (discharge at day 13) and (B) weeks 3–45 after first hospital admission.
We admitted the patient to the cardiology ward with a diagnosis of mRNA vaccine–related myopericarditis. His score on the Naranjo Adverse Drug Reaction Probability Scale was 7, indicating a probable adverse drug reaction to the SARS-CoV-2 vaccine. We considered other causes of myopericarditis, but we thought they were unlikely. For instance, given his lack of infectious symptoms, we did not believe that he had viral myocarditis and we did not order viral serology because of its low utility. We felt that an autoimmune cause such as sarcoidosis was unlikely, given the lack of severe tachyarrhythmias, heart block or heart failure, as well as his normal chest radiograph. Furthermore, he had no clinical features of systemic disease. He did not describe use of any recreational drugs or medications associated with myopericarditis.
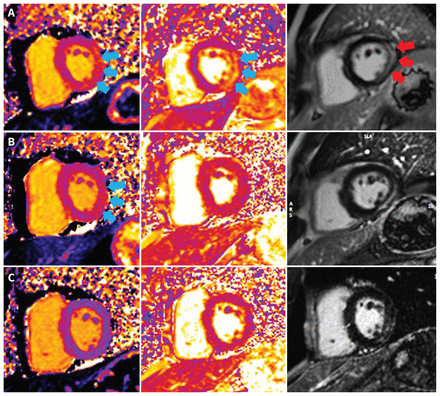
We treated him with acetylsalicylic acid (650 mg, 4 times daily) and colchicine (0.6 mg, twice daily). Cardiac magnetic resonance imaging (MRI) on day 3 of admission showed preserved cardiac function, a left ventricular ejection fraction of 63%, myocardial edema and nonischemic, mid-wall and subepicardial late gadolinium enhancement of the inferolateral wall, consistent with acute myocarditis (Figure 3A). We discharged him after his scan; he had no chest pain and his high-sensitivity troponin I level was 3946 ng/L.
Cardiac magnetic resonance imaging of a 41-year-old man with myocarditis. (A) Short-axis oblique images showing edema of the inferolateral wall on T1 and T2 mapping (blue arrows) (T1 = 1083 [normal 950–1050] ms and T2 = 71 [normal < 57] ms) and on late gadolinium enhancement (LGE) imaging (red arrows) during hospital admission. (B) Persistent myocardial edema in the inferolateral wall on T1 (blue arrows) but not T2 mapping at 2 months (T1 = 1080 ms; T2 = 48 ms). (C) Myocardial edema resolved on T1 and T2 mapping at 6 months (T1 = 1010 ms; T2 = 46 ms). All images were acquired from the same 1.5 T scanner (Aera, Siemens Healthineers).
We prescribed colchicine for 3 months after discharge and a tapering dose of acetylsalicylic acid (decreased by 650 mg/d every week, over 4 weeks). Although he adhered to this medication schedule, he had occasional mild chest pain and his troponin levels were persistently elevated, but much lower than during admission (> 45 ng/L on Siemens assay) ( Figure 2B). A cardiac MRI 3 months after his second SARS-CoV-2 vaccine showed normal left ventricular function, left ventricular ejection fraction of 63%, improving myocardial edema of the inferolateral wall and no new myocardial late gadolinium enhancement (Figure 3B). We started a 6-week course of naproxen (500 mg, twice daily) and extended the colchicine for another 3 months. Three months later, his chest pain symptoms had resolved completely, and his high-sensitivity troponin I was normal (Figure 2B). A follow-up cardiac MRI showed preserved cardiac function with resolution of myocardial edema (Figure 3C). He had upper respiratory tract illnesses 1 year and 14 months after his first SARS-CoV-2 vaccine and tested positive for SARS-CoV-2 by home rapid antigen testing on both occasions (which we presumed to be from the Omicron variant). However, he reported no cardiac symptoms and a repeat test of his high-sensitivity troponin I was normal (Figure 2B).
One year after his initial presentation, he is doing well and has not received further doses of SARS-CoV-2 vaccine.
Discussion
Myopericarditis, myocarditis and pericarditis are rare complications of SARS-CoV-2 mRNA vaccines. According to the United States Centers for Disease Control and Prevention, 1226 probable cases were reported after approximately 300 million SARS-CoV-2 mRNA vaccines were administered through June 11, 2021.1 A systematic review found that mRNA vaccine–related myocarditis typically occurs within 1 week of vaccination and is most common after the second dose.2 A population-based cohort study from British Columbia found that risk is greater among adolescent and young adult male patients (aged 12–29 yr) than older and female patients, and after the mRNA-1273 vaccine, compared with the BNT162b2 vaccine.3 Furthermore, a recent Scandinavian cohort study of more than 23 million patients found that heterologous BNT162b2 and mRNA-1273 dosing was associated with 1.43 myocarditis events per 1000 person-years among males, which was twofold higher than homologous mRNA-1273 dosing and eightfold higher than homologous BNT162b2 dosing.4 The patient we describe received the BNT162b2 vaccine followed by the mRNA-1273 vaccine; myopericarditis was diagnosed 2 weeks after the second vaccine. More evidence regarding the potential risk of heterologous vaccine dosing is needed, particularly among females.
An unusual aspect of this patient’s clinical course is the persistent, subacute myocarditis with elevated serum troponin levels and abnormalities on cardiac MRI up to 4 months after vaccination. In patients with viral myocarditis, myocardial inflammation is caused by direct viral injury and is most evident on cardiac MRI within 2 weeks of symptom onset.5 However, the time course and cause of mRNA vaccine–related myocarditis is not well described. To our knowledge, no reports of persistent troponin elevation and imaging derived myocardial edema have been described. A hypothesis for vaccine-related myopericarditis is that the spike protein and its derivatives are potentially proinflammatory.6 Indeed, circulating exomes containing spike proteins and antibodies to SARS-CoV-2 spike protein can be detected in human plasma 4 months after mRNA vaccination.7 Our patient’s persistent myocarditis may have occurred because of a prolonged autoimmune reaction to spike protein complexes. The lack of apparent myocardial injury after his subsequent SARS-CoV-2 infections may reflect sequence variations in the spike protein gene associated with the Omicron SARS-CoV-2 variant,8 or rapid clearance of viral peptides. Although we cannot exclude community-acquired myopericarditis, this is less likely given the patient’s lack of infectious symptoms during his index event and the temporal relation to his second dose of mRNA vaccine. Furthermore, his score of 7 on the Naranjo Adverse Drug Reaction Probability Scale is consistent with a probable adverse drug reaction.
Treatment of mRNA vaccine–associated myocarditis is usually supportive; consultation with a cardiologist should be considered. Symptoms of pericarditis are treated with nonsteroidal anti-inflammatory drugs, colchicine or both. Patients should be advised to avoid strenuous physical activity and competitive sports for 3–6 months; health care providers should subsequently document the complete resolution of symptoms and diagnostic abnormalities before a return to play.1,9
The National Advisory Committee on Immunization (NACI) recommends against subsequent SARS-CoV-2 vaccination for people who have developed vaccine-associated myocarditis or myopericarditis.10 If, after a risk assessment, the decision is made to administer an additional dose, NACI recommends waiting until the episode of myocarditis has resolved.10
Additional studies are needed on long-term outcomes of mRNA vaccine–related myocarditis; however, short-term prognosis among both adolescent and adult patients with mRNA vaccine–related myocarditis has been favourable.11,12 For example, in 13 patients with vaccine-related myocarditis, myocardial late gadolinium enhancement on cardiac MRI decreased, ejection fraction normalized and no adverse cardiac events were observed after a median follow-up of 159 days.12 The patient we described also had normal cardiac function on follow-up cardiac imaging and has had no cardiac events after the resolution of his myopericarditis. However, he needed prolonged anti-inflammatory treatment given the persistence of symptoms and myocarditis on serial testing.
Footnotes
Competing interests: Ian Paterson reports speaker fees from Pfizer Canada and AstraZeneca. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Kai Wu and Ian Paterson drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/