Pseudallescheria boydii is an environmental fungus implicated as a cause of mycetoma, particularly among patients who are immunocompromised or have structural lung disease.
Mycetoma caused by P. boydii is often misdiagnosed as aspergilloma because of the nonspecific presentations and similarity in radiographic patterns.
Thorough microbiologic workup is needed to diagnose P. boydii infection, with direct microscopic examination of fungal elements and fungal culture as the gold-standard investigations.
Patients with pulmonary pseudallescheriasis have a poor prognosis without treatment; treatment involves surgical resection, with long-term antifungal treatment offered to those who cannot have surgery.
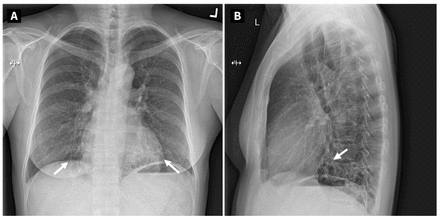
A 48-year-old woman from Southeast Asia was referred to our tertiary hospital after an incidental chest radiograph showed bibasilar bronchiectasis (Figure 1) and 1 of 3 sputum cultures tested positive for Mycobacterium abscessus on routine immigration medical examination. She was a lifelong nonsmoker, and her medical history was notable only for type 2 diabetes that was controlled on oral hypoglycemic agents. She did not have serious cough, sputum production, hemoptysis, dyspnea, or constitutional symptoms. She had no history of previous severe or recurrent pneumonias, symptoms of connective tissue disease, or occupational or environmental exposures.
(A) Posterior–anterior and (B) lateral chest radiographs from a 48-year-old woman with pulmonary pseudallescherioma, depicting cylindrical and cystic bronchiectasis involving both lower lobes (white arrows).
On initial examination, the patient was afebrile with a regular heart rate of 82 beats/min, blood pressure of 115/74 mm Hg, and oxygen saturation of 98% on room air. We heard bibasilar crackles that persisted with deep cough. She had no head or neck lymphadenopathy, nail abnormalities or clubbing, peripheral edema, or skin rash.
To investigate the cause of her bronchiectasis, we ordered a complete blood count with differentials, autoimmune serology for connective tissue disease, an α-1 antitrypsin level for hereditary structural lung disease, immunoglobulin levels and HIV serology for immunodeficiency disorders, a QuantiFERON-TB Gold test for latent tuberculosis infection, and microscopy and cultures for bacteria, fungi, and acid-fast bacilli from sputum samples to test for underlying pulmonary infection (Table 1). Mycobacterium abscessus was again isolated from sputum cultures, and all other investigations were normal.
Initial laboratory and microbiology investigations
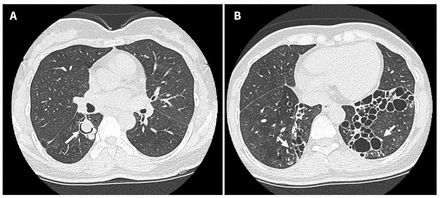
To further characterize the extent and severity of bronchiectasis, we ordered high-resolution computed tomography (CT), which showed extensive bilateral lower lobe varicoid and cystic bronchiectasis, and an incidental internal soft tissue nodule with an air crescent sign in the right lower lobe, which was concerning for a mycetoma (Figure 2). We subsequently performed a bronchoscopy to obtain bronchoalveolar lavage samples, from which Pseudallescheria boydii complex was isolated. Aspergillus cultures and a test for galactomannan from bronchoalveolar lavage fluid were negative.
High-resolution computed tomography scans from a 48-year-old woman with pulmonary pseudallescherioma, showing (A) an intraluminal nodular lesion with air-crescent sign in the subsegmental bronchus of the right lower lobe and (B) severe bibasilar varicoid and cystic bronchiectatic changes, ultimately found to be Pseudallescheria boydii on a bronchoscopy culture.
Considering the microbiology and radiographic findings, we diagnosed pulmonary pseudallescherioma, with bronchiectatic lungs caused by chronic M. abscessus infection. Because pulmonary function tests and radiographic studies showed stable bronchiectasis, our management focused primarily on treating the pulmonary pseudallescheriasis, given the high risk of death with this condition. We observed the M. abscessus infection expectantly. We attempted endobronchial resection of the mycetoma, but this was not feasible because of considerable inflammatory changes in the surrounding tissues leading to the bronchus. We obtained a surgical opinion but, given the patient’s concomitant M. abscessus infection, she was deemed to be at high risk of developing postoperative complications. Therefore, we started medical management of pseudallescheriasis with oral voriconazole (200 mg, twice daily) and monitored her serum drug levels.
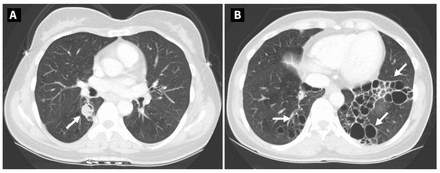
We reattempted endobronchial resection 6 months after starting voriconazole but again could not access the mycetoma. Pseudallescheria was not isolated from the repeat bronchoalveolar lavage, but M. abscessus was still present. We sought a multidisciplinary opinion for definitive management of the mycetoma, including a second surgical opinion. However, given the patient’s relatively asymptomatic state, colonization with M. abscessus, and considerable underlying structural lung disease, the risks of surgical resection of the mycetoma were thought to outweigh the benefits. The patient completed 20 months of oral voriconazole and then transitioned to lifelong suppressive therapy for her pseudallescherioma using itraconazole (200 mg, twice daily). She continued regular chest physiotherapy for airway clearance and received annual influenza vaccines, SARS-CoV-2 booster vaccines, and pneumococcal-23 vaccination to minimize exacerbation of her bronchiectasis. A CT scan obtained 12 months after transition to itraconazole showed that the mycetoma and structural lung disease were stable (Figure 3).
Follow-up computed tomography scans from a 48-year-old woman with pulmonary pseudallescherioma, showing (A) stable mycetoma in the right lower lobe and (B) persistent severe bronchiectasis in the left lower lobe.
Discussion
Pseudallescheria boydii (teleomorph of Scedosporium apiospermum) is a ubiquitous, saprophytic, and filamentous fungus that is an emerging opportunistic pathogen with inherent antifungal resistance.1 It is found in manure-enriched and polluted environments such as industrial areas, decaying and agricultural soil, fertilizers, sewage, and stagnant or polluted water.1 The fungus is present in osmotic environments such as dry bat feces and chicken coops, and even in strictly anaerobic environments such as pond bottoms and submerged wood in estuaries.1 Although P. boydii is rarely isolated from people with no underlying structural lung disease, it is not infrequently isolated from patients with cystic fibrosis, among whom it is the second most common fungal infection after Aspergillus fumigatus, with a prevalence of 5.7%–10%.2
Clinical presentations of P. boydii infection are broad and nonspecific, ranging from asymptomatic colonization of a preexistent pulmonary cavity, cyst, or ectatic bronchus, to locally invasive disease (e.g., saprophytic penetration and destruction of pulmonary tissue, allergic bronchopulmonary pseudallescheriasis, pseudallescherioma or fungus ball) and, at times, systemic invasive infections, particularly among immunocompromised patients (e.g., invasive Pseudallescheria pneumonia, endocarditis, central nervous system and musculoskeletal infection and abscess).1,3 Invasive pulmonary infections by P. boydii among immunocompetent patients have been reported and should be considered in those who have experienced near-drowning or natural disasters, particularly if a clinical suspicion of pneumonia or brain abscess exists.4
Timely identification and differentiation of Pseudallescheria from other fungal pathogens is imperative, as the virulence and antifungal susceptibility profiles differ substantially between organisms. However, diagnosis of Pseudallescheria infection is challenging owing to similar clinical and histopathological features with Aspergillus, Fusarium, and other hyaline hyphomycetes that often cannot be differentiated based on clinical symptomology. Characterization by direct fungal microscopy and culture, and molecular typing of fungal DNA using sequence analysis of internal transcribed spacers or β-tubulin can offer greater diagnostic clarity,1 although these approaches may not be available in routine clinical practice. On chest radiography, pulmonary pseudallescheriasis may present as areas of nodularity, alveolar infiltrates, consolidation, or cavitation.3,5 Similar to invasive aspergilloma, the halo or air-crescent sign, indicative of a fungus ball, can be seen on cross-sectional imaging in pulmonary pseudallescherioma.6 Pseudallescheria and Aspergillus have many clinical, histopathological, and radiographic similarities, but establishing a definitive diagnosis is important because of differences in management and prognosis.
A Pseudallescheria fungus ball, if untreated, can lead to recurrent infections and other complications including hematologic spread and disseminated invasive infections. Surgical débridement or resection is the main therapy for pulmonary pseudallescherioma. Some centres with specialized expertise may offer a less invasive endoscopic removal of intracavitary pulmonary mycetomas for patients who are not candidates for surgery.7
Penetration of the fungus ball by antifungal agents is severely limited in most cases, which can lead to the development of drug resistance, especially over long periods of treatment. Notwithstanding, antifungal treatment should be started in patients whose surgical risk is determined to be higher than the therapeutic benefit (e.g., those with comorbidities, common among patients with Pseudallescheria infection), or if surgical resection is delayed. However, compared with other causes of mycetoma, Pseudallescherioma has high levels of intrinsic resistance to numerous antifungal agents, including amphotericin B, flucytosine, fluconazole, terbinafine, and ketoconazole.1 Moreover, given the absence of therapeutic guidelines, the management of pulmonary pseudallescheriasis — and Pseudallescheria infection in general — is largely based on regional or centre-specific preferences. Accepted regimens include newer triazoles such as voriconazole (which is generally considered a first-line antifungal agent for P. boydii), ravuconazole, isavuconazole, and posaconazole; however, these often have considerable adverse effects.8 Alternatively, some studies have reported effectiveness of itraconazole or echinocandins, including caspofungin, anidulafungin, and micafungin, which are better tolerated.8,9 In our patient, voriconazole monotherapy successfully suppressed the P. boydii infection after a 6-month course. However, because we considered that she was at substantial risk of recurrence and progression to disseminated invasive disease once voriconazole was stopped, we switched her treatment to itraconazole because of its more favourable profile of adverse effects. We expect she will require long-term, likely lifelong, suppressive therapy for pulmonary pseudallescheriasis.
Ultimately, the prognosis of pulmonary pseudallescheriasis is poor. A review of 189 patients with pulmonary pseudallescheriasis reported an overall mortality rate of 26.8% among patients with pseudallescherioma and 57.2% among those with invasive pulmonary pseudallescheriasis.3 When considering all patients, 58.8% who were treated with only antifungal therapy died, compared with 46.2% treated with only surgery or with combination surgery and antifungal therapy. All patients who did not receive any form of therapy died. A more recent review of case reports that included 40 immunocompetent patients with pulmonary pseudallescheriasis suggested an improved overall mortality rate of 12.5%,10 likely in part because of the use of newer antifungal agents, with no significant difference in mortality between those who received surgical or medical management. Given reports of rising rates of fungal disease, particularly among those with structural lung disease after COVID-19 infection,11,12 clinicians should be aware of pulmonary pseudallescheriasis as a cause of mycetoma.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: Christina Thornton reports grants from the CHEST Foundation, Insmed Medical, Trudell Medical and Healthcare Solutions, the University of Calgary, the Cystic Fibrosis Foundation, and Cystic Fibrosis Canada, and travel support from the Cystic Fibrosis Foundation. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/