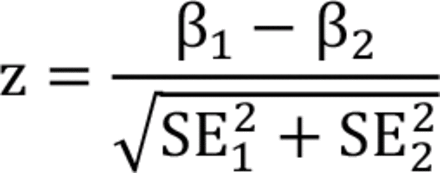Abstract
Background: Few studies have explored the relationship between air pollution and arrhythmia onset at the hourly level. We aimed to examine the association of exposure to air pollution with the onset of acute symptomatic arrhythmia at an hourly level.
Methods: We conducted a nationwide, time-stratified, case–crossover study in China between 2015 and 2021. We obtained hourly information on the onset of symptomatic arrhythmia (including atrial fibrillation, atrial flutter, atrial and ventricular premature beats and supraventricular tachycardia) from the Chinese Cardiovascular Association Database — Chest Pain Center (including 2025 certified hospitals in 322 cities). We obtained data on hourly concentrations of 6 air pollutants from the nearest monitors, including fine particles (PM2.5), coarse particles (PM2.5–10), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone. For each patient, we matched the case period to 3 or 4 control periods during the same hour, day of week, month and year. We used conditional logistic regression models to analyze the data.
Results: We included a total of 190 115 patients with acute onset of symptomatic arrhythmia. Air pollution was associated with increased risk of onset of symptomatic arrhythmia within the first few hours of exposure; this risk attenuated substantially after 24 hours. An interquartile range increase in PM2.5, NO2, SO2 and CO in the first 24 hours after exposure (i.e., lag period 0–24 h) was associated with significantly higher odds of atrial fibrillation (1.7%–3.4%), atrial flutter (8.1%–11.4%) and supraventricular tachycardia (3.4%–8.9%). Exposure to PM2.5–10 was associated with significantly higher odds of atrial flutter (8.7%) and supraventricular tachycardia (5.4%), and exposure to ozone was associated with higher odds of supraventricular tachycardia (3.4%). The exposure–response relationships were approximately linear, without discernible concentration thresholds.
Interpretation: Exposure to air pollution was associated with the onset of symptomatic arrhythmia shortly after exposure. This finding highlights the importance of further reducing air pollution and taking prompt protective measures for susceptible populations during periods of elevated levels of air pollutants.
Arrhythmia is caused by disordered electrical activity in the heart, often occurs abruptly and can lead to severe cardiovascular diseases such as heart failure, acute myocardial infarction, stroke and death.1–3 The Global Burden of Disease study estimated a prevalence of around 59.7 million cases of atrial fibrillation and atrial flutter globally in 2019, and the disability-adjusted life years related to these 2 conditions increased from 3.79 million in 1990 to 8.39 million in 2019.4 Furthermore, the Cardiovascular Health and Diseases Burden Study in China reported that about 4.87 million people had atrial fibrillation in 2019.5 Given the considerable disease burden of arrhythmia, identifying modifiable risk factors is important.
Air pollution has been reported to be a modifiable risk factor for cardiovascular diseases;4,6 therefore, clarifying the effects of air pollution on arrhythmia is important. Air pollution is a complex mixture of particulates and gases that varies by time and location.7,8 Common air pollutants tracked by the World Health Organization (WHO) and government agencies are typically known as “criteria” air pollutants.9–12 Particulate matters in air pollution are broadly categorized by aerodynamic diameter as inhalable particles (< 10 μm, PM10), fine particles (< 2.5 μm, PM2.5) and coarse particles (2.5–10 μm, PM2.5–10). Criteria air pollutants that are gaseous generally include nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3). These pollutants have varied physicochemical properties and have been reported to negatively affect human health in different ways.1,13
Previous epidemiological studies have linked short-term exposure to air pollution with arrhythmia, but the results have been inconsistent.14–21 In addition, most of these studies evaluated the associations only at a daily level;22–24 few have considered the effects of hourly exposure to air pollution before symptom onset. Associations have generally been observed in ecological time-series analyses, leading to difficulty in making causal inferences.23–26 Furthermore, no previous studies have systemically evaluated associations of criteria air pollutants with the acute onset of various types of arrhythmia episodes at a national level.
China has air pollution levels well beyond the WHO guidelines for air quality and those measured in other countries.9,27 Accordingly, we sought to evaluate the association of hourly exposure to air pollution with the acute symptomatic onset of arrhythmia in China.
Methods
Study design and setting
We conducted a time-stratified, case–crossover study at the individual level to evaluate the association between hourly exposure to ambient air pollution and the onset of symptomatic arrhythmia in China among patients admitted to certified chest pain centres from 2015 to 2021.28 This study design has been widely used in epidemiological studies of air pollution.29,30 It allows patients to serve as their own controls, and, therefore, factors that remain relatively stable over a short period (e.g., in the same month) such as sex, age, body mass index, comorbidities and socioeconomic and behavioural factors, can be automatically controlled by design.31,32
We reported this study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for case–control studies.33
Data source
We obtained data for patients with symptomatic arrhythmia from the Chinese Cardiovascular Association (CCA) Database — Chest Pain Center, from Jan. 1, 2015, to Dec. 31, 2021. Established in 2015, this nationwide network uses a standardized registry system to monitor the quality of care provided to patients seen in emergency departments for chest pain or discomfort. The CCA database has been described previously.23,34,35 Several strategies, including an internal reporting checklist, are used to ensure validity and quality of the data (described in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220929/tab-related-content). To ensure data quality, we included only data from hospitals with a chest pain centre certified by the National Chest Pain Center Program.35
Study population and case ascertainment
We extracted medical records for patients admitted to a certified chest pain centre with symptoms (chest pain or discomfort) arising from a primary diagnosis of 1 of 4 main subtypes of arrhythmia — namely, atrial fibrillation, atrial flutter, premature beats (atrial or ventricular in origin) and supraventricular tachycardia — during the study period. We excluded patients with chest pain or discomfort and arrhythmia secondary to another diagnosis (e.g., acute coronary syndrome, pulmonary embolism, aortic dissection). Therefore, we included only those with a principal diagnosis of symptomatic primary arrhythmia in this study.
In these centres, cardiologists diagnose chest pain or discomfort using common clinical practice guidelines and according to symptoms, laboratory biochemical tests and electrophysiological measurements such as electrocardiograms. The principal diagnoses are entered into the database.
Although more specific diagnostic information on the symptomatic arrhythmia is not recorded in the database, only patients with acute symptoms are typically admitted to the chest pain centres. Hence, our study population likely represents patients with a new onset of symptomatic arrhythmia or an acute worsening of pre-existing arrhythmia resulting in chest pain or discomfort, regardless of whether these episodes were diagnosed as intermittent, persistent or permanent.
We excluded patients with symptomatic arrhythmia who did not report the timing of symptom onset, were admitted by referral from other hospitals, or had a second admission of arrhythmia in the same month. We also excluded cases reported by hospitals without nearby air pollution monitoring stations (< 50 km in distance). More information about case ascertainment is described in Appendix 1.
We extracted information on sex, age, self–reported time and location of symptom onset, disease history, principal clinical diagnoses and address of the reporting hospital.
Environmental exposure assessment
We obtained hourly concentrations of 6 criteria air pollutants — PM2.5 (μg/m3), PM10 (μg/m3), NO2 (μg/m3), SO2 (μg/m3), CO (mg/m3) and O3 (μg/m3) — during the study period from the National Urban Air Quality Real–time Publishing Platform. We calculated PM2.5–10 (μg/m3) by subtracting the concentrations of PM2.5 from PM10 at the same station.29
Since the database did not include the location of symptom onset (complete address) for nearly three-quarters (73.2%) of the study population, we used concentrations of air pollutants measured at the nearest monitoring station to the reporting hospital to represent exposure for all participants, a proxy widely used in epidemiological studies of air pollution.29,36 The median distance between the included hospitals and the nearest monitors was 4.4 km (range 0.04–49.9 km).
We obtained hourly ambient temperature and relative humidity for each hospital from the nearest meteorological station (median distance 19.0 km), recorded in the China Meteorological Data Service Center (http://data.cma.cn/). If data at the nearest monitoring station were not available, we used data from the next nearest station.
The final missing rates ranged from 1.1% to 3.1% for various environmental variables; we omitted data from patients with missing environmental variables before statistical descriptions and analyses. To avoid the possible influences of outliers in concentrations of air pollutants, we removed the highest and lowest 2.5% of hourly concentrations during the study period.37
Case and control periods
For our case–crossover study design, we defined the case period as the hour of symptom onset of arrhythmia for each patient. We selected the corresponding control periods for each patient to be in the same hour, day of week, month and year to control for time trends such as circadian rhythms and seasonality.38 For example, if a patient had an onset of symptomatic arrhythmia at 7:00 pm on Monday, Nov. 2, 2020 (defined as the case period), eligible control periods were 7:00 pm on Nov. 9, 16, 23 and 30, 2020. We matched each case period to 3 or 4 control periods, depending on the number of the same days of the week within the month of symptom onset.
Statistical analysis
We used conditional logistic regression models to evaluate the associations between hourly ambient air pollution and arrhythmia onset. Previous studies have shown lagged effects of air pollution on cardiovascular diseases within the first few hours or days after exposure.24,29,39 Therefore, after matching exposure and arrhythmia onset at the hourly level, we considered lag periods of less than 1 day (e.g., 0–6 h, 7–12 h, 13–24 h), and daily lag periods, including 0 days (i.e., lag 0–24 h), 1 day (i.e., lag 25–48 h), 2 days, (i.e., lag 49–72 h) and 3 days (i.e., lag 73–96 h).40
We first hypothesized linear exposure–response relationships by fitting separate models with a linear term for each pollutant at each lag period.24,41 To control for potential confounding by time-varying factors, we included a binary indicator of public holidays and natural cubic splines with 6 and 3 degrees of freedom for temperature and relative humidity, respectively, both of which were included using the 96-hour average (lag 0–3 d), in the models.29 To evaluate possible nonlinear exposure–response relationships, we replaced the linear term for each air pollutant with a natural cubic spline with 3 degrees of freedom. In addition, to determine whether some confounding was present in relation to diurnal or seasonal patterns, we calculated the number of arrhythmia cases and average concentrations of the 6 air pollutants at the hourly or seasonal level, plotted their relationships and reported the Spearman correlation coefficients.
To explore potential effect modifications, we conducted stratified analyses by sex (male and female), age (< 65 and ≥ 65 yr), season and region of the country. We defined the season as warm (April to September) and cold (October to March), and the region as north and south based on the geographic marker of the Qinling–Huaihe line.29
We conducted 2 interaction analyses. First, we added an interaction term between the grouping variable (i.e., sex, age group, region, season) and air pollutants into the main model. Second, we fit separate models in each group and examined the statistical significance of potential effect modifications by 2–sample z tests using the following formula:
where β1 and β2 and were the stratum-specific coefficients of point estimates, and SE and SE2 were the corresponding standard errors. Finally, we conducted a supplementary analysis by adding an interaction term of PM2.5 and O3 into the main model to explore potential interaction effects of air pollutants as reported in previous studies.42–44
We conducted several sensitivity analyses to determine the robustness of the associations between air pollutants and arrhythmia onset. First, to control for potential confounding of coexposures, we fit pairwise 2-pollutant models. Second, we reran the main models separately for patients for whom complete address of symptom onset was available, using exposure data based on both hospital address and onset address. Third, we analyzed the relationship between levels of air pollution and initial visits (before admission) in the chest pain centres (thereafter defined as emergency department visits), matched by the time of first medical contact. Finally, we compared the fit of linear and nonlinear models using likelihood ratio tests, to evaluate the linear assumption used in the main analyses.45
All analyses were 2 sided, with an α level of 0.05. We presented results as percent change and 95% confidence intervals (CIs) for the risk of arrhythmia onset associated with an interquartile range (IQR) or a 10 μg/m3 (1 mg/m3 for CO) increase in concentrations of air pollutants.29 To correct for multiple testing, we used the Benjamini–Hochberg procedure for the set of 24 (6 pollutants × 4 arrhythmia subtypes) main analyses and the set of 48 (6 pollutants × 8 subgroups) subgroup analyses, with a false discovery rate of 0.05.46 More information on our analysis methods is available in Appendix 1.
We performed all analyses in R (version 3.6.3, R Project for Statistical Computing) with the survival package for conditional logistic regression.
Ethics approval
The study protocol was approved by the Institutional Review Board of the School of Public Health, Fudan University (no. 2021–04–0889). All data were anonymized, with access to the database authorized by the CCA Database — Chest Pain Center.
Results
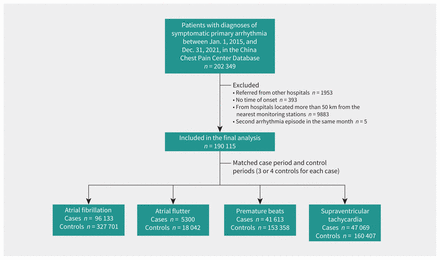
We included a total of 190 115 patients with symptomatic primary arrhythmia in this analysis, of whom 96 133 had atrial fibrillation, 5300 had atrial flutter, 41 613 had overall premature beats (including atrial or ventricular) and 47 069 had supraventricular tachycardia (Figure 1). The mean age of the study participants was 64 (range 18–98) years, with 47.1% younger than 65 years. Demographic characteristics are summarized in Table 1. We included data from 2025 certified hospitals in 322 Chinese cities. These hospitals are most densely distributed in the eastern region of the country, followed by the central region and the western region, a distribution that is largely consistent with socioeconomic and air pollution characteristics in China (Appendix 2, Supplemental Figures S1 and S2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220929/tab-related-content).
Flow chart.
Patient demographic characteristics
During the study period, the 24-hour average concentrations of PM2.5, PM2.5–10, NO2, SO2, CO and O3 were 34.4 μg/m3, 25.5 μg/m3, 27.5 μg/m3, 8.9 μg/m3, 0.7 mg/m3 and 59.0 μg/m3, respectively. The 24-hour average temperature was 17.5°C and the relative humidity was 71.4% (Table 2). We found weak-to-moderate correlations between air pollutants and meteorological conditions, with Spearman coefficients ranging from –0.45 to 0.63 (Appendix 2, Supplemental Table S1).
Descriptive statistics of air pollutants and meteorological factors over the 24 hours before arrhythmia onset
Diurnal and seasonal patterns
At the diurnal level, we did not observe any significant correlations between hourly concentrations of air pollutants and hourly numbers of arrhythmia onset (Appendix 2, Supplemental Figure S3 and Table S2). Likewise, no significant relationships were found between average concentrations of air pollution and numbers of arrhythmia cases at the seasonal level (Appendix 2, Supplemental Figure S4 and Table S2).
Lag period patterns
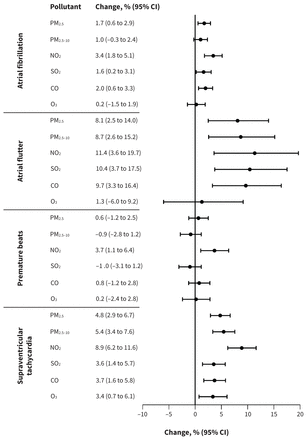
Short-term exposure to ambient air pollution was associated with higher risk of the onset of symptomatic arrhythmia, but the magnitude differed by lag period. Generally, the effects occurred during the first several hours and either persisted or attenuated thereafter; exposures at a lag period of 0 days (0–24 h) showed the highest risks compared with other daily lags. The risks attenuated greatly after 24 hours, except the association between exposure to PM2.5 and atrial flutter, which was strongest at lag 2 days (Appendix 2, Supplemental Figures S5–8). Therefore, we reported results primarily using a lag period of 0 days in subsequent analyses (Figure 2).
Change in the odds of arrhythmia onset per interquartile range increase in concentration of air pollutants. The exposure duration was an average lag period of 0–24 hours preceding the onset for all analyses other than the analysis for PM2.5 and atrial flutter, which used a lag period of 49–72 hours. Note: CI = confidence interval, CO = carbon monoxide, NO2 = nitrogen dioxide, O3 = ozone, PM2.5 = particulate matter with an aerodynamic diameter ≤ 2.5 μm, PM2.5–10 = particulate matter with an aerodynamic diameter between 2.5 and 10 μm, SO2 = sulfur dioxide.
Associations between specific air pollutants and arrhythmia subtypes
Exposure to ambient air pollution showed stronger associations with atrial flutter and supraventricular tachycardia than the other 2 subtypes. At lag periods of 0–24 hours, exposure to PM2.5, NO2, SO2 and CO was associated with the onset of atrial fibrillation, atrial flutter and supraventricular tachycardia. Exposure to PM2.5–10 showed positive and significant associations with atrial flutter and supraventricular tachycardia at lag 0 days. We found no evidence of an association between the pollutants and onset of premature beats except for NO2. At lag periods of 0–24 hours, exposure to O3 was associated only with supraventricular tachycardia (Figure 2 and Appendix 2, Supplemental Figures S5–8).
Among the relationships of the pollutants with onset of different types of arrhythmias, NO2 consistently showed the strongest associations. Specifically, an IQR increase in the concentration of NO2 was associated with increases in the odds of atrial fibrillation (3.4%, 95% CI 1.8% to 5.1%), atrial flutter (11.4%, 95% CI 3.6% to 19.7%), premature beats (3.7%, 95% CI 1.1% to 6.4%) and supraventricular tachycardia (8.9%, 95% CI 6.2% to 11.6%) (Figure 2). The effect estimates per 10 μg/m3 (per 1 mg/m3 for CO) increase in concentration of air pollutants at a lag period of 0 days are presented in Table 3.
Change in the odds of onset of symptomatic arrhythmia associated with a 10 μg/m3 (1 mg/m3 for CO) increase in air pollutant concentrations during lag 0 to 24 hours
The curves depicting the exposure–response relationship between all air pollutants and arrhythmia onset increased monotonically and were, in general, approximately linear, without any apparent concentration thresholds (Figure 3 and Appendix 2, Supplemental Figures S9–10). Furthermore, we found no statistically significant differences between linear models and nonlinear models (Appendix 2, Supplemental Table S3).
Exposure–response curves for the concentrations of 6 air pollutants and atrial fibrillation (panels A–F) and supraventricular tachycardia (panels G–L). The associations are presented as percentage change in the odds of the outcome in comparison to the odds at the median concentration over a lag period of 0–24 hours. The solid red lines represent the point estimates, and the intervals between dashed red lines represent 95% confidence intervals (CIs). The other dashed lines represent the concentration of air pollutants (green = 25th percentile, blue = 75th percentile, black = 95th percentile). Note: CO = carbon monoxide, NO2 = nitrogen dioxide, O3 = ozone, PM2.5, = particulate matter with an aerodynamic diameter ≤ 2.5 μm, PM2.5–10 = particulate matter with an aerodynamic diameter between 2.5 and 10 μm, SO2 = sulfur dioxide.
Stratified and interaction analyses
Stratified analyses showed that the associations of the 6 pollutants with overall onset of arrhythmia was stronger among patients who were male or younger than 65 years. In addition, exposure to air pollution exposure had stronger associations among patients located in the north region and during the cold season (Appendix 2, Supplemental Table S4).
Analyses for the inclusion of interaction terms showed some significant effect modifications. Specifically, higher risks of arrythmia onset were found among males (significant difference for SO2), patients younger than 65 years (significant difference for PM2.5–10), patients located in the north region (significant difference for SO2) and during the cold season (significant difference for all air pollutants) (Appendix 2, Supplemental Tables S5). We did not find any statistically significant interactions between PM2.5 and O3 for the 4 types of arrhythmias (Appendix 2, Supplemental Table S6).
Sensitivity analyses
Analyses using 2-pollutant models showed similar results after simultaneously controlling for co-pollutants (Appendix 2, Supplemental Figures S11–14). Furthermore, for patients who provided complete onset address (n = 51 331), the estimates were not significantly different in analyses using exposures assigned according to the onset address and those assigned according to the hospital address (Appendix 2, Supplemental Table S7). Compared with the main analyses, we observed a slightly stronger association between exposure to air pollutants and arrythmia onset using exposures matched by the time of initial visit to the emergency department (Appendix 2, Supplemental Table S8). Finally, the estimated associations between exposure to air pollutants and arrhythmia onset (including subgroup analyses) remained statistically significant after we corrected for multiple testing (Appendix 2, Supplemental Table S9–10).
Interpretation
We evaluated the association between hourly exposure to air pollution (PM2.5, PM2.5–10, NO2, SO2, CO and O3) and symptom onset of 4 common arrhythmias, and found that acute exposure to ambient air pollution was associated with increased risk of symptomatic arrhythmia. The risks occurred during the first several hours after exposure but attenuated greatly 24 hours after exposure. Among the 6 pollutants, NO2 showed the strongest association with all 4 arrhythmias, while O3 was associated only with supraventricular tachycardia. We did not observe any concentration thresholds for the exposure–response relationships.
Previous findings on the association of air pollutants with arrhythmia have been mixed.24,26,47,48 Most studies were subject to the ecological fallacy (the bias that may occur when associations that exist between variables at the aggregate level may not represent the true associations that exist at the individual level),49,50 and were limited in sample size, geographical coverage and time specification.21,23,24,26 Our case–crossover study strengthens previous findings by providing large-scale, individual-level evidence.
Some studies have shown positive associations between exposure to PM (PM2.5 and PM10) and risk of arrhythmia, but results have varied in different countries and regions.51,52 Consistent with previous studies,15,53,54 we found that exposure to PM2.5 increased the risk of arrhythmia. We also found that PM2.5–10 increased the risk of atrial flutter and supraventricular tachycardia but had little effect on atrial fibrillation and premature beats.
The existing evidence on the association of gaseous pollutants with arrhythmia is also inconsistent.15,55–60 Several studies have reported no association,14,54,60 but we found NO2 had the strongest associations with arrhythmia, compared with other pollutants. The inconsistency of these findings may be explained by the difference in air pollution mixture, climate conditions and population susceptibility, as well as the study design, statistical power and exposure lags that were used in the various studies.21,47,48
We found the strongest associations of air pollution with atrial flutter and supraventricular tachycardia, followed by atrial fibrillation and premature beats. Although the exact mechanisms are not yet fully understood, the association between air pollution and acute onset of arrhythmia that we observed are biologically plausible. Some evidence has indicated that air pollution alters cardiac electrophysiological activities by inducing oxidative stress and systemic inflammation, affecting multiple membrane channels, as well as impairing autonomic nervous function.1,61–65
Among numerous epidemiological studies of air pollution, few have explored the interactive effects for 2 or more air pollutants, likely because of intractable problems in relation to multi-collinearity and exposure measurement. The interactive effects of PM and O3 have been most commonly investigated in previous studies but the results have been mixed.42,43,66,67 For example, a time-series study from Shanghai reported that higher levels of PM10 strengthened the association between exposure to O3 and mortality.66 Another study from Moscow also observed increased mortality from all causes, ischemic heart disease and cerebrovascular disease, associated with exposure to PM10 at concurrent higher levels of O3.67 In contrast, a study from Hong Kong observed that exposure to O3 may alleviate the adverse effects of particulate air pollution on cardiovascular and respiratory morbidity; 42 another study in Seoul also observed attenuated associations of exposure to O3 with stroke mortality in conjunction with higher levels of PM10.43 Our study did not find any statistically significant interactions between PM2.5 and O3 for all types of arrhythmias, and further studies are warranted to evaluate these possible interactions.
Given the nature of our database and study design, the observed triggering effect of air pollution could be the result of an acute worsening or a recurrent episode of a pre-existing arrhythmia (including a previously undiagnosed arrhythmia) induced by exposure to pollutants within 24 hours. Some previous studies linking short-term air pollution exposure and arrhythmia have reported comparable effect sizes to our study.21,24,47,48 For example, Halldorsdottir and colleagues21 conducted a time-stratified, case–crossover study in Reykjavik, and found that an increase in NO2 of 10 μg/m3 increased the risk of atrial fibrillation at a lag period of 0 days (odds ratio [OR] 1.03, 95% CI 1.01 to 1.05). Another study observed that an IQR increase in PM2.5 levels was associated with an increased risk of atrial fibrillation (OR 1.05, 95% CI 1.01 to 1.09) at a lag of 48–72 hours.488
These effects appeared to be appreciably smaller than those of other well-known and established risk factors for arrhythmia. For example, the Framingham Heart Study and 2 other systematic reviews indicated that the prevalence of diabetes, obesity and some cardiovascular diseases (e.g., hypertension, congestive heart failure, valve disease) was associated with increased risk of developing atrial fibrillation, with hazard ratios (or risk ratios) ranging from 1.2 to 5.9,68–70 which were about 1–6 times greater than those associated with air pollution. Despite the smaller effect sizes associated with exposure to air pollution, the disease burden could still be considerable because of the ubiquitous exposure.
Stratified analyses in our study showed that the association of exposure to air pollutants and arrhythmia was stronger among male patients, which is consistent with previous studies.23,71 It is unclear why male patients may be more vulnerable, but this may be owing to a higher prevalence of lifestyle-related risk factors for arrhythmia (e.g., smoking, alcohol intake) or more exposure to air pollution through outdoor activities, including work.7 Furthermore, the associations were stronger in the cold season, which may be owing to a higher level of pollution during this period from central heating in China.72 In addition, low temperature has been found to enhance the harmful effects of air pollution on the cardiovascular system.29
In previous studies, older adults have generally been found to be more vulnerable to air pollution;29,45 however, we found that older patients had lower risks of arrhythmia related to air pollution. We postulate that this may be a result of a blunted response to exposure to air pollution from pre-existing diseases or age-related impairment.73
Understanding the timing of exposure and symptom onset is important from a clinical and public health perspective. However, previous evidence on sensitive exposure windows was uncertain.14,41,74 We found evidence on lag patterns, which indicated that air pollution was associated with the onset of symptomatic arrhythmia within the first hours of exposure.
We observed approximately linear exposure–response relationships, without apparent thresholds, which was similar to previous results.24,41 The State of Global Air 2020 study27 and another previous study75 reported that the annual average level of PM2.5 in China was 31.4 μg/m3 in 2019, which is much higher than in Canada (7.1 μg/m3),27 and the WHO guidelines for global air quality (5 μg/m3).9 Although our study was conducted in China, where exposure levels are very high, the results may still be relevant to other countries because of the “no threshold” findings we observed in the exposure–response relationships. Our findings also highlight the necessity of more stringent air pollution control, as well as prompt responses for susceptible populations during episodes of air pollution.
Limitations
As with most previous epidemiological studies on short-term health effects of air pollution,29,48 we obtained all exposure data from fixed site monitors nearest to the reporting hospitals; consequently, errors in exposure measurement were inevitable. However, the patients in our study were usually taken to the nearest hospital for timely treatment, and the resultant non-differential misclassification could only cause Berkson bias, which would not influence the mean estimates of the associations but could lead to an inflation of confidence intervals.36,76
We studied patients with symptomatic arrhythmia who visited participating chest pain centres and, thus, we did not include those who had asymptomatic arrhythmia, were admitted by other hospital departments or died before arriving at hospital. In addition, the database we used did not collect information on patients with ventricular tachycardia, ventricular flutter or ventricular fibrillation. Therefore, our population may not be a representative sample of patients with arrythmia in China, and our results may have limited generalizability to other regions. The lack of detailed information on disease history and diagnosis prevented us from conducting some clinically important stratified analyses.
We cannot exclude the possibility of diagnostic or reporting errors in such a nationwide analysis. We deemed such errors to be random and not related to variations in air pollution, so they were not likely to bias our results. The varying sample size for different subtypes of arrhythmia attenuated the comparability in their associations with air pollution. Finally, although we used a time-stratified, case–crossover study design, we could not fully exclude residual confounding, especially from time-varying, individual risk factors (such as emotional shock and strenuous exercise).
Conclusion
Acute exposure to ambient air pollution was associated with increased risks of symptomatic arrhythmia. The risks occurred during the first several hours after exposure but attenuated greatly after 24 hours. The exposure–response relationships were, in general, approximately linear without discernable thresholds of concentrations of air pollutants, but the magnitude varied by type of air pollutant, arrhythmia subtype, subpopulation and geographic or seasonal features. Our study adds to evidence of adverse cardiovascular effects of air pollution, highlighting the importance of further reducing exposure to air pollution and of prompt protection of susceptible populations worldwide.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Junbo Ge, Yong Huo and Renjie Chen jointly contributed to the conception, design and supervision of the work. Huichu Li, Yixuan Jiang, Junbo Ge, Yong Huo and Renjie Chen contributed to study methodology. Xiaowei Xue, Jialu Hu, Dingcheng Xiang, Weiyi Fang, Hongbing Yan, Jiyan Chen, Weiming Wang, Xi Su, Bo Yu, Yan Wang, Yawei Xu, Lefeng Wang, Chunjie Li, Yundai Chen and Dong Zhao contributed to the acquisition, analysis and interpretation of data. Xiaowei Xue, Jialu Hu and Dingcheng Xiang drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Xiaowei Xue, Jialu Hu and Dingcheng Xiang contributed equally to this work.
Data sharing: The China Chest Pain Center data contains sensitive data and cannot be shared via public deposition because of information governance restrictions in place to protect individuals’ confidentiality. Access to data for external researchers (not affiliated with the Chinese Cardiovascular Association) requires researchers to be physically based in the institute. Access to data is available only once approval has been obtained through the individual constituent entities controlling access to the data.
Funding: This work was supported by the National Key Research and Development Program (2022YFC3702701), Shanghai Committee of Science and Technology (21TQ015), the National Natural Science Foundation of China (92043301) and the Shanghai Clinical Research Center for Interventional Medicine (19MC1910300).
- Accepted April 3, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/