Abstract
Background: Chronic obstructive pulmonary disease (COPD) is common among surgical patients, and patients with COPD have higher risk for complications and death within 30 days after surgery. We sought to describe the longer-term postoperative survival and costs of patients with COPD compared with those without COPD within 1 year after inpatient elective surgery.
Methods: In this retrospective population-based cohort study, we used linked health administrative databases to identify all patients undergoing inpatient elective surgery in Ontario, Canada, from 2005 to 2019. We ascertained COPD status using validated definitions. We followed participants for 1 year after surgery to evaluate survival and costs to the health system. We quantified the association of COPD with survival (Cox proportional hazards models) and costs (linear regression model with log-transformed costs) with partial adjustment (for sociodemographic factors and procedure type) and full adjustment (also adjusting for comorbidities). We assessed for effect modification by frailty, cancer and procedure type.
Results: We included 932 616 patients, of whom 170 482 (18%) had COPD. With respect to association with risk of death, COPD had a partially adjusted hazard ratio (HR) of 1.61 (95% confidence interval [CI] 1.58–1.64), and a fully adjusted HR of 1.26 (95% CI 1.24–1.29). With respect to impact on health system costs, COPD was associated with a partially adjusted relative increase of 13.1% (95% CI 12.7%–13.4%), and an increase of 4.6% (95% CI 4.3%–5.0%) with full adjustment. Frailty, cancer and procedure type (such as orthopedic and lower abdominal surgery) modified the association between COPD and outcomes.
Interpretation: Patients with COPD have decreased survival and increased costs in the year after surgery. Frailty, cancer and the type of surgical procedure modified associations between COPD and outcomes, and must be considered when risk-stratifying surgical patients with COPD.
Contemporary estimates suggest that more than 10% of surgical patients have COPD.1 Patients with COPD are at increased risk for complications and death within 30 days after surgery;2–4 previous work estimates a 35% increase in odds of morbidity and a 30% increase in odds of death attributable to COPD after risk adjustment.3 However, existing studies have substantial shortcomings. Several included select hospitals, which limits generalizability, while others were narrow in scope and studied selected surgical procedures; most did not follow up patients for more than 30 days after surgery.2–7
Patients with COPD may be at increased risk over the longer term owing to age and other comorbidities.8,9 Understanding the longer-term outcomes of surgical patients with COPD is critically important to accurately guide informed consent discussions and project care needs. The costs to health systems to care for patients with COPD after surgery are also unknown;10 delineating these costs would facilitate system-level budgeting and resource allocation. We sought to compare survival and health care costs up to 1 year after inpatient elective surgery between patients with and without COPD in a large, real-world surgical population in a health system where hospital and physician care are government-funded.
Methods
Study design and setting
We conducted a population-based cohort study in Ontario, Canada, which we report according to the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) checklist.11
Data sources
In this study, we used several databases linked on an individual level, including the Discharge Abstract Database (DAD), which captures hospital admissions and discharges, diagnoses and procedures; the Ontario Health Insurance Plan (OHIP) Database, which captures physician claims; the National Ambulatory Care Reporting System, which captures emergency care; the Continuing Care Reporting System, which captures long-term and respite care; and the Registered Persons Database (RPDB), which captures deaths. These data sets were linked using unique encoded identifiers and analyzed at ICES.12,13 ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement.12
Participants
We included all patients aged 35 years or older living in Ontario who underwent certain intermediate-to-high-risk elective non-cardiac surgeries from April 2005 to March 2019, including carotid endarterectomy, open or endovascular abdominal aortic aneurysm repair, peripheral arterial bypass, total hip replacement, total knee replacement, shoulder surgery, large bowel surgery, partial liver resection, pancreaticoduodenectomy, gastrectomy, esophagectomy, nephrectomy, cystectomy, prostatectomy or hysterectomy (see Appendix 1, Supplement 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220733/tab-related-content, for rationale for selected procedures and procedure codes). 14–16 We included only the first surgery for patients who underwent multiple procedures. To reduce the probability of complicated surgeries that required revision or reoperation influencing our findings, we excluded patients who had undergone the same type of surgery in the previous year. We also excluded patients who were ineligible for OHIP, and those who had undergone lung transplant or surgery for lung volume reduction.
Exposure
The primary exposure was physician-diagnosed COPD, defined as 1 or more ambulatory claims or 1 or more hospital admissions for COPD any time before the index surgery date.17,18 This definition was previously validated against an expert panel in respiratory medicine and yielded a sensitivity of 85.0%, a specificity of 78.4% and a positive predictive value of 81.3%. In sensitivity analyses, we used a specific definition of COPD, defined as 3 or more ambulatory claims for COPD within any 2-year period or 1 or more COPD hospital admissions (sensitivity 57.4%, specificity 95.4%).17
Outcomes
Our primary outcome was all-cause death in the year after surgery; the secondary outcome was total health care costs in the year after surgery. We identified deaths during the index hospital admission from the DAD, and deaths after discharge using the RPDB. We calculated total health care costs from the perspective of a single-payer health system using established methods for case-costing with data from Ontario’s administrative databases, standardized to 2019 Canadian dollars (Appendix 1, Supplement 2). We summed costs from the index hospital admission and from the year after discharge to generate total health care costs per patient. Censoring in cost analyses was negligible over the study period; total costs were available for living patients for the year after surgery. We captured the following costs using validated methods: costs for acute hospital admissions and emergency department visits using the resource intensity weight method; costs of complex continuing care and long-term care based on resource utilization groups and length of stay; physician service costs based on fee-for-service payments to the physician; rehabilitation costs based on the rehabilitation patient group case mix and length of stay; and costs for home care based on the number and costs per visit.19,20
Covariates
We identified demographics (age, sex) from the RPDB. We assigned income based on median neighbourhood income from Census data, and rural status based on the Rurality Index of Ontario.21,22 We used validated algorithms to determine congestive heart failure, hypertension or diabetes mellitus.23–25 We used International Classification of Disease, Tenth Revision diagnostic codes in the DAD in the 3 years before the index surgery to determine coronary artery disease, atrial fibrillation, cerebrovascular disease, primary lung cancer, primary non-lung cancer, peripheral vascular disease, chronic kidney disease and liver disease (see Appendix 1, Supplement 3 for comorbidity codes).26–28 We extracted type and site of the surgical procedure from the DAD, which has high accuracy. 14,15 We determined frailty using the preoperative frailty index, a multidimensional index modelled after the original Canadian Study of Health and Aging Frailty Index; values greater than 0.21 were indicative of frailty.29 We used the Johns Hopkins Adjusted Clinical Group (ACG) System (version 10) to generate clinically oriented categories of morbidity, generated by summing aggregated diagnosis groups (ADGs), operationalized as low (scores 0–5), medium (5–9) or high (≥ 10).30,31 These measures capture overall burden of comorbidity and are associated with health resource use in various health systems.32–34 We excluded COPD status in the generation of frailty and Johns Hopkins ACG scores.
Statistical analysis
We compared characteristics of people with and without COPD using standardized differences. Standardized differences of less than 10% represent small differences.35
We generated cumulative mortality rates by COPD status using the Kaplan–Meier method. We computed unadjusted hazard ratios (HRs) to evaluate the impact of COPD on survival using Cox regression models. We generated both partially adjusted (adjusting for sociodemographic factors, including age, sex, income quintile, rural residence and type of surgery) and fully adjusted models (adjusted for sociodemographic factors, as well as comorbidities, frailty and level of comorbidity).16,26,28,36 Age was a continuous variable, expressed as a 4-knot restricted cubic spline to account for any nonlinear association with the outcome. We modelled surgery as a 7-level categorical variable that included type (e.g., minimally invasive) and site (e.g., upper abdominal) based on clinical sensibility.37 The proportional hazards assumption was supported by nonsignificant correlations between Schoenfeld residuals with transformed time.
We calculated health care costs by COPD status; we also plotted unadjusted total costs by COPD status for each type of surgical procedure. We used regression analyses to assess relative incremental costs associated with COPD. We ran ordinary least squares regression of the logarithmic transformation of costs (see cost modelling approach in Appendix 1, Supplement 2).38 In multivariable analyses, we adjusted for the same covariates as the survival model. We verified underlying model assumptions.
We used R (version 3.4.2) for analyses. An α value of 0.05 was used as the level of significance.
Effect modification analysis
As frailty may modify the association between COPD and outcomes, 39 we evaluated its potential effect modification by including an interaction term between COPD and frailty, with no changes to remaining covariates. Similarly, as respiratory complications commonly occur after open upper abdominal surgery and open aortic surgery,40,41 we evaluated effect modification with an interaction term between COPD and procedure type. We separately evaluated potential effect modification by cancer by including an interaction term between COPD and cancer. We used the Wald method to assess the presence of interaction.
Sensitivity analysis
To account for possible misclassification of the exposure variable, we conducted sensitivity analyses using the more specific, previously described definition of COPD.17 We also expressed COPD as a 3-level variable (no COPD, incident COPD within 5 years of diagnosis, prevalent COPD of greater than 5 years’ duration) to evaluate whether duration of COPD affected association with outcomes. We conducted analyses with follow-up limited to 30 days after surgery to evaluate the influence of COPD on perioperative outcomes. Finally, we conducted a sensitivity analysis restricting procedures to those completed during a more contemporary period from 2014 to 2019.
Sample size and missing data
As this was a population-based study, we included all eligible patients; no formal sample size calculation was used. Data were missing for only 0.3% of participants for neighbourhood income quintile, and 0.08% of participants for rural residence. Given negligible missing data, we conducted a complete case analysis.
Ethics approval
The study was approved by the University of Toronto Research Ethics Board (Protocol #36261).
Results
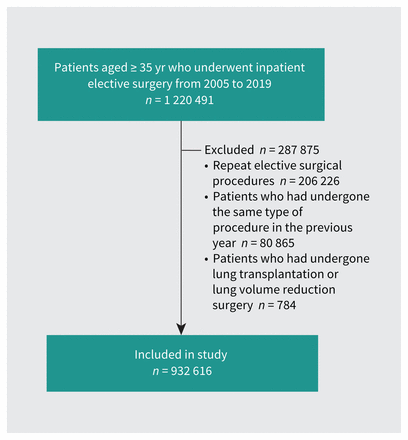
Of 1 220 491 patients aged 35 years or older who underwent inpatient elective surgery in Ontario from 2005 to 2019, we included 932 616 patients (Figure 1), of whom 170 482 (18.3%) had physician-diagnosed COPD.
Cohort creation.
Table 1 shows patient characteristics by COPD status. Patients with COPD were older and were more frequently male, of lower income quintile, residents of long-term care and admitted to hospital before surgery than those without COPD. They were also more likely to have other pre-existing diseases such as coronary artery disease, congestive heart failure and lung cancer. A greater proportion of patients with COPD had frailty and medium-to-high comorbidity. Patients with COPD more frequently underwent orthopedic surgery, open upper abdominal surgery and vascular surgery.
Characteristics of people with and without COPD who underwent inpatient elective surgery in Ontario, Canada, from 2005 to 2019
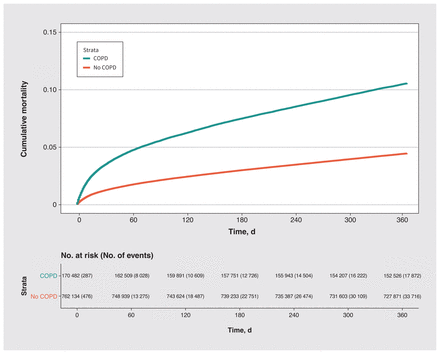
During the year after surgery, 52 021 (5.6%) patients died, including 18 007 (10.6%) patients with COPD and 34 014 (4.5%) patients without COPD. Within 30 days after surgery, patients with COPD were more likely to die (n = 5873, 3.4%) than those without (n = 9429, 1.2%). Unadjusted cumulative mortality by COPD status is presented in Figure 2. Patients with COPD had an increased risk of all-cause death (Table 2), with an unadjusted HR of 2.45 (95% CI 2.41–2.50), a partially adjusted HR of 1.61 (95% CI 1.58–1.64), and a fully adjusted HR of 1.26 (95% CI 1.24–1.29).
Unadjusted cumulative mortality over a year after surgery by chronic obstructive pulmonary disease (COPD) status among patients who underwent inpatient elective surgery.
Unadjusted, partially adjusted and fully adjusted associations of COPD with 1-year survival and costs after inpatient elective surgery*
The median costs of caring for patients with and without COPD in the year after surgery are presented in Table 3. Compared with patients without COPD, unadjusted costs were 38.7% (95% CI 38.2%–39.3%) higher among patients with COPD, including 21.7% (95% CI 21.3%–22.0%) during the index hospital admission and 46.8% (95% CI 45.5–48.2) in the year after surgery. Patients with COPD had 13.1% (95% CI 12.7%–13.4%) higher total costs with partial adjustment and 4.6% (95% CI 4.3%–5.0%) higher total costs with full adjustment, which was similar to higher costs in the year after surgery (15.0% [95% CI 14.0%–16.1%] with partial adjustment and 4.9% [95% CI 3.9%–5.8%] with full adjustment).
Costs for the total period, the index hospital admission and the year after hospital admission, as well as unadjusted, partially adjusted and fully adjusted estimates of relative increase for patients with COPD versus those without COPD*
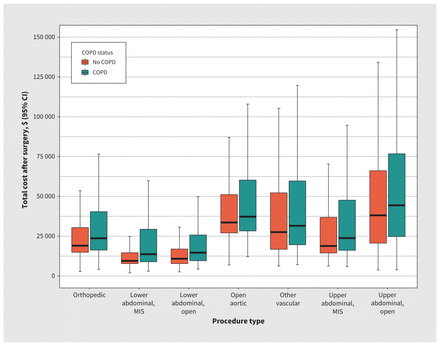
Unadjusted total costs after each type of surgery by COPD status are presented in Figure 3. Certain surgical procedures, such as upper abdominal surgery and aortic surgery, were associated with greater total costs, regardless of COPD status. The impact of COPD on costs varied by procedure type and was more pronounced after lower abdominal and orthopedic surgery. Estimates of absolute cost increases attributable to COPD, with partial and full adjustment, over a range of non-COPD costs, as well as the proportion of patients by COPD status that accrued total costs greater than $15 000 in the year after surgery, are presented in Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220733/tab-related-content.
Unadjusted total cost over a year after surgery for each procedure type by chronic obstructive pulmonary disease (COPD) status among patients who underwent inpatient elective surgery. Note: CI = confidence interval, MIS = minimally invasive surgery.
Effect modification analysis
We found evidence of significant interaction between COPD and frailty, COPD and cancer, and COPD and procedure type (HRs for unadjusted, partially adjusted and fully adjusted analyses are presented in Table 4).
Assessment of effect modification by frailty, cancer and surgical procedure on the association between COPD and outcomes*
Sensitivity analysis
Findings were robust in sensitivity analyses (Table 2). When COPD was based on a more specific definition (n = 71 986 with COPD) or a 3-level definition (n = 56 761 with incident COPD, n = 113 721 with prevalent COPD), and in separate analyses restricted to a contemporary period (n = 61 246 patients with COPD of total n = 329 836), observed associations between COPD status with death and cost in unadjusted analyses diminished in magnitude after risk adjustment. Notably, the HRs for death (partially adjusted HR 2.02 [95% CI 1.98–2.07]; fully adjusted HR 1.40 [95% CI 1.37–1.43]) and relative change in cost (partially adjusted increase of 25.6% [95% CI 25.0%–26.2%]; fully adjusted increase of 9.8% [95% CI 9.3%–10.3%]) were higher when COPD status was ascertained using a more specific definition. In sensitivity analyses with a 30-day follow-up period, associations of COPD with death were greater than in the primary analysis, but also diminished with risk adjustment (partially adjusted HR 1.72 [95% CI 1.66–1.78]; fully adjusted HR 1.32 [95% CI 1.28–1.37]). The magnitude of associations with cost over 30 days were smaller than in the primary analysis (partially adjusted relative increase 7.0% [95% CI 6.7%–7.3%], fully adjusted increase of 2.1% [95% CI 1.8%–2.3%]).
Interpretation
Patients with COPD had lower survival and greater health care costs in the year after scheduled surgery than patients without COPD. The condition was associated with a 61% increase in hazard of death and 13% greater total costs after adjustment for sociodemographic factors and procedure type. After also adjusting for comorbidities, COPD was associated with a 21% increase in hazard of death and 5% greater total costs. Frailty, cancer and type of surgical procedure modified associations between COPD and outcomes.
Nearly 1 in 5 surgical patients had physician-diagnosed COPD. Recent evidence suggests prolonged time to functional recovery after major surgery among older patients with comorbid illness, which would include patients with COPD.42,43 In our study, patients with COPD were at increased risk of death and accrued greater costs beyond 30 days after surgery. Studies looking at a follow-up period of 30 days after surgery may not capture the overall burden of impaired recovery in complex surgical patients.
Patients with COPD typically have concurrent comorbidity, biopsychosocial issues and frailty.44 For example, 50% of patients with COPD have a high burden of comorbidity, and almost 1 in 5 live with frailty. Disentangling the direct effects of COPD, associated comorbidity or frailty on patients’ outcomes is challenging. Our findings highlight the importance of careful risk prediction and decision-making for patients with COPD who are considering surgery.
The causal pathways between coexisting conditions are complex and can be difficult to temporally define for each individual, meaning that typical approaches to adjustment, such as multivariable regression, may not fully capture the overall impact of each comorbidity. Acknowledging these limitations, our unadjusted analysis showed large effect sizes related to COPD, but sequential adjustment for comorbidities, oncologic status and frailty substantially attenuated these associations. Although patients with COPD are at elevated risk for adverse postoperative outcomes, the magnitude of this risk that is explained by COPD itself, as opposed to concomitant comorbidity and frailty, appears to be relatively small.
From a practical perspective, determining the exact magnitude of elevated risk attributable to COPD has limited value for patients and clinicians. Clinicians should identify the presence of COPD as an indicator of risk and complexity, which should trigger further assessment for other meaningful and modifiable preoperative risk factors, including comorbidity, frailty and type of surgery. Procedures such as open aortic and upper abdominal surgery are associated with higher postoperative risks irrespective of COPD status,40,41,45 whereas others, such as orthopedic and lower abdominal surgery, may be of significantly greater risk for patients with COPD. Much like a recent evaluation of patients with frailty,43 our results suggest that perioperative management of patients with COPD requires careful consideration of the multiple domains that contribute to their elevated perioperative risk.
Our findings of the greater costs accrued by COPD patients after surgery will facilitate system-level planning by hospital administrators and policy-makers. Importantly, the increased cost burden occurred in the period after hospital admission, suggesting that these patients warrant attention for additional follow-up and rehabilitation beyond 30 days after surgery. The impact of COPD was also heterogeneous across different types of surgery, with greater observed impact after lower abdominal and orthopedic surgery procedures. Future work is needed to identify which facets of patients’ long-term recovery require further investment after different types of surgery to improve processes of care and long-term outcomes in patients with COPD.
Limitations
We defined our cohort by identifying patients who underwent surgery, giving rise to possible selection bias as patients with more severe disease may not have been offered surgery. Although this is a possibility, no current perioperative evidence precludes elective noncardiothoracic surgery owing to COPD alone.46 Nonetheless, the generalizability of our results to patients who are not surgical candidates may be limited. Also, should evidence from our study change risk prediction and surgical decision-making, our results may not be generalizable to future patients undergoing surgery. Our study did not include a measure of COPD severity. We conducted sensitivity analyses using duration of COPD to account for incident versus prevalent COPD, and using a more specific definition of COPD; the latter showed a higher risk of death and greater costs among patients with COPD, although magnitudes of unadjusted associations still diminished with risk adjustment. Future work is needed to generate a severe COPD definition using health administrative data that predicts increased risk of postoperative complications.
Conclusion
Chronic obstructive pulmonary disease is commonly encountered among patients undergoing inpatient elective surgery. Patients with COPD have higher costs and risk of death when undergoing surgery than patients who are otherwise similar. The association between COPD and adverse outcomes persists beyond the immediate perioperative period, and is affected by frailty, cancer and type of surgery. Perioperative patient care should include comprehensive assessment and treatment tailored not only to COPD, but also to management of concomitant conditions and surgical disease.
Footnotes
Competing interests: Daniel McIsaac reports participation on the data safety monitoring board of the PRICE-2 trial. Andrea Gershon reports funding from the Canadian Institutes of Health Research and PSI Foundation, as well as a stipend from Novartis. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Ashwin Sankar, Kevin Thorpe, Daniel McIsaac, Duminda Wijeysundera and Andrea Gershon conceived the study. Ashwin Sankar, Jin Luo and Andrea Gershon acquired data. All of the authors analyzed and interpreted the data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by the Government of Ontario. Duminda Wijeysundera is supported in part by a Merit Award from the Department of Anesthesiology and Pain Medicine at the University of Toronto, and by the Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital and the University of Toronto. Andrea Gershon is funded by a PSI Graham Farquharson Knowledge Translation Fellowship.
Data sharing: Data may be obtained from a third party and are not publicly available. The data set from this study is held securely in coded form at ICES. Although data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministries of Health (MOH) and of Long-Term Care (MLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH or MLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information and Ontario Health. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
- Accepted November 7, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/