Abstract
Background: The MySafe program provides pharmaceutical-grade opioids to participants with opioid use disorder via a biometric dispensing machine. The objectives of this study were to examine facilitators and barriers to safer supply via the MySafe program and the associated outcomes.
Methods: We conducted semistructured interviews with participants who had been enrolled in the MySafe program for at least a month at 1 of 3 sites in Vancouver. We developed the interview guide in consultation with a community advisory board. Interviews focused on context of substance use and overdose risk, enrolment motivations, program access and functionality, and outcomes. We integrated case study and grounded theory methodologies, and used both conventional and directed content analyses to guide inductive and deductive coding processes.
Results: We interviewed 46 participants. Characteristics that facilitated use of the program included accessibility and choice, a lack of consequences for missing doses, nonwitnessed dosing, judgment-free services and an ability to accumulate doses. Barriers included technological issues with the dispensing machine, dosing challenges and prescriptions being tied to individual machines. Participant-reported outcomes included reduced use of illicit drugs, decreased overdose risk, positive financial impacts and improvements in health and well-being.
Interpretation: Participants perceived that the MySafe program reduced drug-related harms and promoted positive outcomes. This service delivery model may be able to circumvent barriers that exist at other safer opioid supply programs and may enable access to safer supply in settings where programs may otherwise be limited.
Canada continues to deal with an ongoing overdose crisis, largely driven by fentanyl and its related analogues in the unregulated drug supply, with 7902 reported deaths from opioid overdose in Canada in 2021.1 Clinicians, people who use drugs, researchers and advocates alike have called for bold action to address these preventable deaths. In an era where retention rates for traditional programs of opioid agonist therapy are extremely low,2 safer supply — a harm reduction intervention intended to reduce the risks of illicit drug use — is regarded as a promising, albeit controversial, intervention that targets those at highest risk of overdose.3–9 Most safer supply programs provide patients with pharmaceutical-grade opioids of known quantity and quality as an alternative to the toxic, illegal drug supply.10,11 Eligibility varies across programs, but generally includes people with opioid use disorders who use street-purchased, illicit fentanyl, people who frequently experience overdose or, more broadly, those considered at high overdose risk. Currently, we know of no programs in Canada that allow youth to enroll and, overall, access to safer supply remains limited nationally. Several models of safer supply programs have been implemented in select cities across Canada, including overdose prevention site-and clinic-based witnessed dosing programs;4,12,13 programs that provide take-away doses;3,14 those integrated within supportive housing, with on-site primary care clinics that provide delivery to participants’ rooms for nonwitnessed use;15 and programs that provide take-away doses via biometric opioid dispensing machines;5 we focus on the latter in this study.
The MySafe program started in Vancouver, Canada, and uses secure biometric dispensing machines to administer tablet hydromorphone daily to patients who have a history of overdose, are regularly using opioids and have fentanyl detected in their urine drug screens.5 No research to date has examined the program’s accessibility, uptake and associated outcomes. We sought to examine facilitators and barriers to accessing safer supply via MySafe’s biometric opioid dispensing machine and the associated outcomes. The specific study objectives were to examine site-specific social, structural and physical contextual factors that affect program access and uptake; assess general satisfaction and determine areas for improvement of the MySafe program (including medication reception and adherence; staff, site and machine interactions; and medication dispensing model); and examine program effects on participants’ health and well-being.
Methods
Study design and setting
This study is part of a larger, mixed methods, longitudinal evaluation of the MySafe program (detailed in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221550/tab-related-content). We undertook an applied health research approach to generate meaningful data to inform safer supply policies and programs.16 This approach was informed by an integrative methodology of case study and grounded theory.17–19 Case studies have predefined boundaries and focus on the successes or failures of a particular intervention, and on understanding contextual factors that affect cases.17 In case studies, questions are formulated and data are coded based on existing literature and expertise from the field (deductive);17 grounded theory extended our approach to include inductive reasoning to identify emergent categories.18
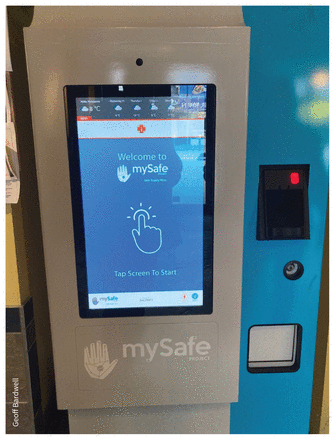
Medications for MySafe participants are dispensed at a local pharmacy, and the prescribed doses (as packaged, daily doses of tablets) are manually inserted into the machine. The machine (Figure 1) scans a participant’s handprint and then dispenses their daily prescription (e.g., 1 package of 16 hydromorphone tablets). Participants are expected to take their medications daily. Participants undergo a medical evaluation before enrolment and agree to regular follow-ups (at 1, 6 and 12 months) with a health professional to monitor health outcomes and conduct urine drug screens at the discretion of MySafe staff (e.g., with dose increases, to assess medication use). The initial dose is determined by the prescribing physician, along with participants, and titrated to a suitable dose based on an individual’s need. Dose adjustments normally occur during the first month of enrolment; however, dosing changes can be requested at any point. At the time of data collection, the prescribing physician did the medical assessment and follow-ups, monitored the drug screens and supervised staff to provide ongoing support. During data collection, the program existed in an overdose prevention site in Vancouver (accessible 8 am to 9 pm) and in 3 supportive housing buildings, 2 in Vancouver and 1 in Victoria, where the machines are accessible 24 hours a day; participants did not need to live in the buildings to access the machines.20 MySafe staff are available on site, primarily during day-time hours, and refill the machine regularly to prevent interruptions in medication delivery. Overnight staff at housing sites are trained on basic troubleshooting (e.g., rebooting the machine); when complex issues arise, MySafe staff respond as soon as possible during daytime hours.
MySafe biometric dispensing machine.
Image courtesy of Geoff Bardwell
Data generation and analysis
We conducted qualitative, 1-to-1, semistructured interviews with program participants and used purposive sampling to ensure representation from across the 3 MySafe sites in Vancouver. Eligibility required participants to be enrolled in the program for at least 1 month to ensure they were able to provide accounts of recurring program experiences. Data collection occurred between November 2021 and April 2022. Program staff recruited potential participants during their daily work duties, which included engagement with program participants. Members of the research team conducted interviews on site in private rooms. Participants provided written informed consent and were provided with an honorarium of $30.
We developed interview guides in consultation with a community advisory board comprising people with lived or living experience of drug use and other relevant stakeholders (e.g., clinical staff). We developed a draft guide and shared it at an in-person meeting with the community advisory board. The group read through each section of the guide to add relevant questions, make edits and ensure the questions were comprehensible. This process allowed us to avoid pilot-testing as we did not encounter any major issues with the interview guide. The final interview guide is in Appendix 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221550/tab-related-content. A research coordinator and a postdoctoral fellow — both with extensive training in qualitative interviewing — conducted the interviews. Interviews focused on the context of drug use and overdose risk, enrolment motivations, program access and machine functionality, and program outcomes. We reached data saturation before ending interviews;21 however, we interviewed additional participants as a means of providing an opportunity to give voice to marginalized individuals.22 Three team members (A.I., G.B., M.M.) held meetings regularly during data collection and analysis phases to ascertain any similarities, differences and emerging patterns in an iterative process.23 We used conceptual depth criteria to determine saturation, which requires a range of evidence, including frequency of occurrences, connected concepts across the interviews and validity via resonance with existing literature.24
Interviews were transcribed by a professional transcription service. Each team member reviewed several interview transcripts individually, and then we met as a group to discuss both a priori and emergent concepts and categories to focus our coding framework.18,25 We used content analysis to identify patterns, with a focus on prevalence to identify dominant categories.25 To account for our integrated methodological approach, we conducted both a conventional content analysis (i.e., inductive coding) as well as a directed content analysis (i.e., deductive coding based on pre-established categories).25 Using NVivo 12, 2 team members (A.I., M.M.) completed coding, with supervisory support (G.B.). We determined pre-established categories based on findings from existing studies, study objectives and the authors’ expertise in the field.18 These categories included perceived overdose risk, overdose prevention, privacy, accessibility and nonwitnessed ingestion.13,15,26 Given that safer supply is a harm reduction intervention, our interpretation of the findings was informed by harm reduction approaches to substance use. In addition, the authors’ positionalities as substance use scholars and clinicians, with experience working in and studying harm reduction interventions, affected our analysis.27 Taking a team-based approach allowed for a collaborative reflexivity whereby authors were able to discuss the findings and challenge individual interpretations.28
Ethics approval
We obtained ethics approval from the University of British Columbia and Providence Health Care Research Ethics Board. Given the sensitive nature of the topic, individual demographic information (e.g., age, gender) is not provided after each quote.
Results
We enrolled 46 participants, of whom 14 identified as women, 13 as Indigenous and 32 as White, with a median age of 41 (range 25–68) years (Table 1). Most (n = 36) lived in supportive housing, with 20 receiving opioid agonist therapies. Participants reported using a range of drugs in the previous 30 days, including heroin or fentanyl (n = 40), crystal methamphetamine (n = 32), cannabis (n = 27), crack cocaine (n = 20), benzodiazepines (n = 13), alcohol (n = 13), cocaine (n = 10) and other opioids (n = 10).
Participant demographics
Facilitators
Accessibility and choice
All participants described the convenience and ease of accessing their safer supply from a biometric dispensing machine. Participants enrolled at housing sites reported how 24-hour access provided them with flexibility in terms of when they could safely access their medication, and how this was preferential to other programs with set time frames for medication access, such as pharmacies or scheduled delivery (Table 2, participant 43). Those who did not live on site also had 24-hour access, as staff were always available to provide access to the dispensing machine. Most participants who were enrolled at the overdose prevention site (accessible 8 am to 9 pm) described the convenience of its proximity to where they resided. Participants also emphasized how program accessibility enhanced their autonomy, commenting on the ability to choose the time and frequency of engagement with no repercussions; they compared these benefits with negative experiences in other clinical or pharmacy settings or opioid agonist therapy programs, such as judgment from clinicians or others (Table 2, participant 32), or strict policies around medication access and consequences for missing doses (Table 2, participant 31).
Participant quotations regarding facilitators
Nonwitnessed use
Unlike many other programs for safer supply and opioid agonist therapy, MySafe did not require clinician-witnessed dosing. Participants described their experiences in other programs and how taking medication such as methadone in front of others (e.g., pharmacists, pharmacy customers) led to embarrassment and shame. Participants explained that the MySafe program provided a sense of privacy and was free of judgment, which encouraged program engagement (Table 2, participants 31 and 32). The lack of restrictions not only provided participants with more independence in terms of where and when they took their medication, but also what consumption modes they used to administer it (Table 2, participant 35). Participants reported various modes of consuming the hydromorphone tablets (e.g., injection, oral, intranasal), with use depending on context for some participants (e.g., oral in public, injecting in private settings or at an overdose prevention site).
Contingency planning
Given the greater independence provided to patients via the policy of nonwitnessed use, participants described an ability to stockpile their medications. Having a surplus of medications was useful for those who wanted to leave the city (e.g., vacation; Table 2, participants 3 and 47). Participants also saved some of their medication in case they were unable to attend the program or to save for days when they used a higher dosage, limiting their need to seek out illicit drugs (Table 2, participant 33). Lastly, participants described accumulating pills in case the machine broke, leaving them unable to access their daily safer supply, which could lead them to use illegal drugs (Table 2, participant 1).
Barriers
Technological issues
Almost every participant (n = 40) described having some sort of technical difficulty when using the MySafe machine. Issues included frozen screen or error messages (Table 3, participant 1), problems scanning palm prints (Table 3, participant 3) or finding the machine damaged by an external party. Technological errors sometimes led participants to either go into withdrawal or have to buy drugs from the illegal market (Table 3, participant 44), thereby increasing their overdose risk and affecting their daily routines. Depending on the time of day, MySafe staff were usually available to provide medications to participants or to reboot the machines (Table 3, participant 55). Most participants reported these issues as being infrequent (e.g., once a month), although 1 participant described having weekly problems scanning into the machine, reportedly because of having calloused or damaged palms from manual labour.
Participant quotations regarding barriers
Dosing challenges
Participants reported that their medications did not match the current strength of illicit opioids, particularly for those who had been using unregulated opioids (e.g., heroin, fentanyl) for many years and had a very high tolerance (Table 3, participant 4). Some described not being prescribed a dosage that they desired and how dosing limits were not realistic to adequately manage withdrawal (Table 3, participant 32). Given that some participants had issues managing their withdrawal solely with medications provided by the MySafe program, they sought out additional opioids elsewhere, including diverted prescription pills (Table 3, participant 13).
Dedicated machine
About one-third of participants described issues with their prescriptions being tied to a specific machine. If participants were registered to a machine in their supportive housing building, they were unable to also be registered to the machine at the overdose prevention site and vice versa (Table 3, participant 3). Participants discussed how this required them to be near the machine daily, which affected their mobility and where they chose to live and work (Table 3, participant 32).
Participant-reported outcomes
Reduced use of illicit drugs
Although some participants reported dosing challenges, and almost all reported using illicit drugs concurrently, most participants reported reducing their use of street-purchased drugs since enrolment (Table 4, participant 51). Responses varied, with some participants reporting substantial reductions in use of illicit drugs and others suggesting they had been spending less money on drugs. Among these participants, having regular access to hydromorphone tablets allowed them to substitute illicit drugs with a prescribed alternative (Table 4, participant 1).
Participant quotations regarding participant-reported outcomes
The frequency with which program participants used illicit street-purchased drugs while accessing MySafe varied. Some participants described using illicit drugs less frequently (Table 4, participant 39); others reported still using street-purchased drugs daily but using smaller amounts than before program enrolment (Table 4, participant 42). A few participants reported no longer using street-purchased drugs since enrolling in the MySafe program (Table 4, participant 32). For these participants, the safer supply medications were sufficient to meet their needs (e.g., withdrawal management, euphoria) without them having to supplement with street-purchased drugs.
Decreased overdose risk
Given that most participants were supplementing their MySafe medications with street-purchased drugs, some participants reported experiencing an overdose since enrolling in the program. However, others expressed that the program was successful in reducing overdose risk, recounting frequent overdose experiences before enrolment (Table 4, participant 38). Participants explained that, by providing them with a regulated (and thus, safer) opioid supply, the program was reducing their need to use toxic drugs, thereby mitigating their risk of overdose (Table 4, participant 5).
Participants also spoke about how the medications were safer when compared with the unregulated and highly variable illicit supply. They explained that, for example, when purchasing fentanyl on the street, they never knew how strong the drugs were going to be, and that the hydromorphone received from the MySafe program provided reassurance that they were using safer drugs of known potency (Table 4, participant 50).
Financial impacts
Participants described various financial impacts since program enrolment. For many participants, having regular access to hydromorphone tablets reduced their spending on illicit drugs. Participants described having more money or spending substantially less on illicit drugs since enrolling in the program, and reported being able to buy other items such as food and clothes (Table 4, participant 5). In comparison, many participants described spending most or all of their money on drugs before enrolling in the MySafe program, often engaging in criminalized or stigmatized forms of income generation (e.g., sex work, panhandling, robbery or theft, drug selling) to procure drugs. For many, this was a constant cycle of seeking money and then spending time to find and purchase small amounts of drugs (Table 4, participant 13 and 47). Several participants reported no longer having to engage in these forms of income generation since enrolling in the program (Table 4, participant 47 and 2).
Improvements to health and well-being
Most participants discussed how the MySafe program was having a positive impact on their health and well-being. Responses were diverse, and included mental health improvements (e.g., less stress, better mood; Table 4, participant 6), improved interpersonal relationships, having more free time, being more productive or functional, dietary improvements and health service engagement (Table 4, participant 32). Many also spoke about using the program to manage withdrawal symptoms, something that was described as difficult before enrolling in the program (Table 4, participant 5). Participants explained that being able to manage withdrawal through the program with regular and low-barrier access to hydromorphone further reduced their need to seek out illicit drugs (Table 4, participant 50).
Interpretation
Participants enrolled in the MySafe program described a variety of facilitators and barriers to program access and engagement. Facilitators included accessibility and choice, nonwitnessed dosing, a lack of consequences for missing doses, a judgment-free setting and an ability to accumulate doses as contingency plans (e.g., for travel). Barriers included technological issues with the machine, dosing challenges and prescriptions being tied to individual machines. Participants reported reduced use of illicit drugs, decreased overdose risk, financial improvements and improvements to health and well-being. Taken together, these findings illustrate promising aspects of, and areas for improvement to, the MySafe model of safer supply.
Our findings add to a small but emerging body of research on safer supply programs in Canada that reports how these programs have the potential to reduce overdose risk by limiting illicit opioid exposure,3,15,29–31 with 1 study reporting no opioid-related deaths among program participants3 and another reporting 0 overdoses among program participants.31 As most participants in this study reported using fewer illicit drugs and described reductions in overdose risk since enrolling in MySafe, our findings provide further support of the potential that safer supply programs may offer to address overdose risk. Our findings also illustrate how the MySafe program provides secondary benefits beyond the intended program outcomes (e.g., reduction in overdose risk), addressing physical, mental and social well-being. Given the known associations between sociostructural factors and overdose risk,32–35 our findings underscore the importance of addressing issues attendant to drug use and overdose vulnerability, and are in line with previous research showing the feasibility of safer supply programs to address matters at the intersection of drug use, drug market volatility and social determinants of health.3,13,15,36
Little research has examined barriers and facilitators to engagement in and adherence to safer supply programs.13,37 A recent study reported benefits of accessing pharmaceutical alternatives, including increased agency regarding how participants consumed their drugs and when they chose to attend the clinic.13 However, participants also described barriers, including limited hours of operation, the need to attend the clinic several times a day and nurse-witnessed ingestion.13 These findings are similar to studies on opioid agonist therapy that report how stigma and programmatic restrictions constrain initiation and retention.2,38–41 Our study findings suggest that the MySafe program circumvents these barriers by providing 24-hour access (or 13-hour access, for the overdose prevention site) and not requiring witnessed ingestion. Integrating the MySafe program in supportive housing allowed greater ease of access to residents, which is particularly important, given the reported links between housing and overdose42,43 and calls for targeted interventions in housing environments where people are most at risk.26,34,44 In addition, this program appears to have potential to limit exposure to violence that is associated with procuring drugs from the illegal market, although further research is needed to confirm such impacts.45
The MySafe program was not without its issues. Technological issues were described by most participants, resulting in some having withdrawal symptoms and others seeking illicit opioids when unable to access medications from the machine. However, many participants reported accumulating their prescriptions for circumstances when they were not able to use the machine, such as when away on vacation or when technological issues arose. A lack of takeaway doses has been described as a barrier in studies on access to opioid agonist therapy.40,46–48 In the case of the MySafe program, however, patients should not have to stockpile their medications because of technological issues; this could lead to intentional or unintentional diversion of medications.
A problem confronting all programs of safer supply and opioid agonist therapy in the current era of high-potency illicit drugs is addressing illicit fentanyl-induced withdrawal and the inability of previously sufficient dosages of pharmaceutical opioids to provide appropriate withdrawal management or anti-craving effects. Similar to our results, insufficient dosing of opioid agonist therapy has been found to shape continued use of illicit drugs.49,50 A recent study on adherence to safer supply opioids found that 60-day adherence was higher for those receiving higher daily doses.37 Dosing challenges therefore need to be addressed, which may include increasing the maximum daily dose or providing medications other than hydromorphone, such as prescription fentanyl or diacetylmorphine.4,51,52 However, this limitation speaks more to available medications and less of the MySafe model itself. Clinical guidelines that detail how to address dosing challenges in safer supply programs are urgently needed, including how and when to increase the maximum daily dosages of hydromorphone or provide access to alternative opioid medications.
Our findings suggest that the MySafe model could be beneficial in other settings, particularly in jurisdictions with challenges in accessing safer supply, including rural and remote communities with geographical and transportation barriers and in pharmacies that are under-resourced and have limited hours of operation. 53,54 In addition, this model shows promise for medication delivery beyond safer supply and could include opioid agonist therapy, direct-acting antiviral tablets or other medications that are commonly accessed by marginalized groups. This would be especially beneficial for structurally vulnerable populations who have compounding barriers when accessing services related to substance use (e.g., Indigenous and racialized communities, sex workers, gender and sexual minorities). Future research is needed to assess the feasibility of the MySafe program in other communities, as well as to explore opportunities to emulate this model for the safe supply of other medications.
Limitations
We conducted this study in Vancouver, which is a unique context in terms of the prevalence of drug use, the population density of people who use drugs (particularly in the Downtown Eastside) and the availability and concentration of related services. Therefore, application of these findings to other settings may be limited. Although we recruited diverse participants, their experiences may not reflect those of all MySafe program participants, nor of people receiving safer supply medications from other programs and models. We may not have captured other outcomes and program engagement factors (e.g., changes in technology, illicit drug supply, funding, policies and regulations, staffing, program locations). Additional longitudinal research is therefore needed to understand barriers, facilitators and outcomes of this program over time. The eligibility criteria (enrolment for at least 1 month) meant we did not capture the experiences of anyone who may have stopped participating in the program shortly after enrolment. Although we presented preliminary findings to MySafe Society, greater inclusion of the community advisory board at this stage may have strengthened validity of the results. Outcomes were self-reported and have not been confirmed via objective measures. However, research has shown both the reliability and validity of self-reported data among people who use drugs.55,56 Nonetheless, future research is needed, including mixed-methods approaches to confirm outcomes of safer supply programs.
Conclusion
We examined participant perspectives on outcomes, barriers and facilitators of the MySafe program. Although some challenges are apparent, this program shows promise in providing patients with a low-barrier and accessible means to receive a safer opioid supply, which they perceived to lead to a variety of positive outcomes. Given the urgency of the overdose epidemic, novel interventions must be implemented and evaluated to address risk of overdose and death. Continued reliance on traditional treatments (e.g., opioid agonist therapy, abstinence-based programs) is insufficient to address the overdose crisis. Our study shows one such intervention that can be used as a low-barrier model for the delivery of safer supply programs in settings contending with high rates of overdose death.
Acknowledgements
This study took place on the unceded territories of the xwməθkwəy̓əm (Musqueam), Skwxwú7mesh (Squamish), and selílwitulh (Tsleil-waututh) Nations. The authors thank the participants for their contributions to this study. The authors would also like to express sincere thanks to the MySafe community advisory board and acknowledge MySafe Society staff across the sites who supported recruitment efforts. The MySafe machines were supplied and supported by Dispension Industries.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Geoff Bardwell and Thomas Kerr acquired funding and supervised this study. Geoff Bardwell, Andrew Ivsins and Thomas Kerr contributed to the conception and design of the study. Andrew Ivsins and Manal Mansoor contributed to acquisition of data. Geoff Bardwell, Andrew Ivsins and Manal Mansoor contributed to analysis of data. Geoff Bardwell and Andrew Ivsins drafted the manuscript. All of the authors contributed to interpretation of data, revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Geoff Bardwell and Andrew Ivsins contributed equally to this work (shared first authorship).
Data sharing: The qualitative data set for this study is not publicly available, given the sensitive nature of the topic, including confidential information that could compromise confidentiality and consent.
Funding: This study was supported by funding from Health Canada’s Substance Use and Addictions Program (2021-HQ-000128). The funding source did not play a role in the design of the study, execution, data interpretation and publication.
- Accepted April 11, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/