Major hemorrhage is a challenging clinical problem that can occur with traumatic injury, postpartum hemorrhage, major surgery and gastrointestinal bleeding.
A massive hemorrhage protocol (MHP) should be activated for patients with uncontrolled hemorrhage who meet clinical activation criteria and are expected to need multiple blood products, in addition to red blood cells.
Tranexamic acid should be considered for patients with major bleeding.
When the MHP is activated, plasma should be given at a 2-to-1 ratio of red blood cells to plasma; plasma transfusion should subsequently be guided by the results of coagulation tests.
Platelet transfusion and fibrinogen replacement should be guided by the results of laboratory tests.
Laboratory testing should be done every hour during active resuscitation to allow for goal-directed administration of blood products.
Major hemorrhage is life-threatening and can occur in a variety of clinical settings. For the purpose of this review, we defined major hemorrhage as life-threatening bleeding that is likely to result in the need for massive transfusion (i.e., ≥ 10 units of red blood cells in 24 h).1,2 We did not use the commonly used definition of major bleeding from the International Society on Thrombosis and Haemostasis because some bleeding events in the score do not need transfusion (e.g., intraocular or intra-articular bleeding, a 20 g/L decrease in hemoglobin levels).3 Management of major hemorrhage is most challenging in rural settings where the availability of blood products and specialized laboratory tests are limited. The response to major hemorrhage is labour-intensive and requires the support of a multidisciplinary team.
Best practice for patients with major hemorrhage is now informed by numerous randomized controlled trials (RCT), as shown in Table 1, which allowed the development of standardized massive hemorrhage protocols (MHPs). However, implementation of and adherence to MHPs has been a challenge. We review the evidence for management of major hemorrhage and MHPs to support the implementation of such protocols at all hospital types to optimize the care of patients with major hemorrhage (Box 1).17,18
Key randomized controlled trials to inform the clinical management of patients with a major hemorrhage
Box 1: Literature search
We searched PubMed from January 2010 to December 2022 for large (> 100 patients) randomized controlled trials involving patients with major hemorrhage due to traumatic injury, complications of pregnancy, gastrointestinal bleeding or a surgical procedure. We also included relevant systematic reviews of randomized trials and published guidelines for the management of major bleeding in these settings.
What is a massive hemorrhage protocol?
An MHP is a standardized and evidence-based approach to the management of major hemorrhage. The protocol specifies activation criteria and process of activation (phone or electronic), how blood products are provided (e.g., automatically released in bundles), type and frequency of laboratory testing, when to transfuse, when to administer tranexamic acid (TXA) and criteria for termination of the MHP. An MHP needs to be in place at any organization that maintains an inventory of red blood cells or that staffs an emergency department, critical care unit, labour and delivery unit or operating room.19 Achieving prompt control of the source of hemorrhage, obtaining dual intravenous access (18 gauge or larger) and considering permissive hypotension until source control is obtained in select patients (e.g., those with penetrating injury without head injury)20 are crucial elements. The MHP should be designed to minimize phone calls to the blood bank that interrupt and delay the immunohematology technologist as they crossmatch blood, thaw plasma and prepare fibrinogen concentrate. Poor adherence to MHPs is associated with inferior patient outcomes.17,19 The aggressive replacement of blood is considered to counteract the acute coagulopathy of trauma that leads to profound hypofibrinogenemia.21
When should a massive hemorrhage protocol be activated?
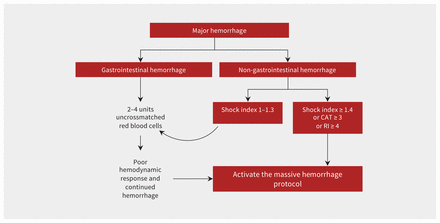
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support, in addition to red blood cells. When faced with a hemorrhaging patient, the clinical team must decide whether to activate the MHP or request individual blood products (e.g., 2–4 units of uncrossmatched red blood cells). Overactivation of MHPs is a major burden on blood bank technologists and the blood supply as it can result in high rates of blood wastage. In addition, not all bleeding episodes are sufficiently severe to warrant blood transfusion and, in such cases, it is appropriate to commence resuscitation with crystalloid alone. Some experts have suggested the use of 2 units of uncrossmatched blood before the MHP is activated to minimize the burdens of overactivation.22
No criteria or bleeding score at which activation of an MHP is warranted has universal agreement. A systematic review identified 24 potential tools for predicting which patients need MHP activation.23 Many tools are applicable only to patients with traumatic injury, consist of multiple variables or require advanced mathematical computation but, for trauma patients, a shock index (heart rate divided by systolic blood pressure) greater than 1 is more sensitive than more complex trauma scores (e.g., Assessment of Blood Consumption Score) and can be readily applied to most bleeding patients.24 Other options evaluated in trauma include a Critical Administration Threshold (CAT) score or a Resuscitation Intensity (RI) score.25 A CAT score of 3 or higher (CAT3+) is defined as the need for transfusion of 3 or more red blood cells in the first hour of resuscitation. An RI score of 4 or higher (RI4+) is defined as the need for 4 or more of any of the following in the first 30 minutes: each unit of blood (red cell, plasma or platelet), 500 mL of colloid or 1000 mL of crystalloid.
No activation tools have been validated for nontrauma patients. For patients with gastrointestinal hemorrhage, activation of an MHP should be discouraged as these patients can usually be managed with crystalloid support or transfusion of red blood cells alone. In a cluster RCT of red blood cell transfusion in patients with gastrointestinal hemorrhage in the United Kingdom (TRIGGER trial), only about 5% of the 936 patients enrolled required plasma, platelet or fibrinogen replacement.26
All hospitals must have policies for rapid release of uncrossmatched, group O red blood cells. A suggested decision-making algorithm for protocol activation is shown in Figure 1.
Algorithm to guide activation of the massive hemorrhage protocol in patients with major hemorrhage. A Critical Administration Threshold (CAT) score of 3 or higher (CAT 3+) is defined as the need for transfusion of 3 or more red blood cells in the first hour of resuscitation. A Resuscitation Intensity (RI) score of 4 or higher is defined as the need for 4 or more of any of the following in the first 30 minutes: unit of blood (red cell, plasma or platelet), 500 mL of colloid or 1000 mL of crystalloid. The shock index is calculated as the heart rate divided by systolic blood pressure.
Who should receive tranexamic acid?
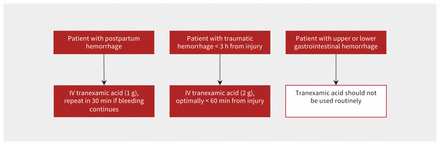
The administration of TXA to patients with major hemorrhage has been studied extensively (Table 1). It should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown. A suggested approach is shown in Figure 2. In the CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2) study, trauma patients were randomized to receive TXA (1 g bolus, and then 1 g infused over 8 h) or matching placebo.4 Tranexamic acid reduced the risk of all-cause death and death from hemorrhage, without an increase in thromboembolic complications. A benefit was seen only among patients administered the drug within 3 hours of injury, with the benefit most pronounced when administered within 60 minutes (relative risk [RR] of death 0.68, 95% confidence interval [CI] 0.57–0.82). In the STAAMP (Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport) trial, trauma patients were randomized to receive either 1 g of TXA or placebo before arriving at the hospital.9 On arrival to hospital, TXA-treated patients were randomized to receive either placebo, 1 g of TXA or 2 g of TXA. The greatest reduction in 30-day mortality was seen in the subgroups of patients treated within 60 minutes of trauma, those who received a total of 3 g of TXA or those who had severe shock (systolic blood pressure < 70 mm Hg). To reduce the complexity of care, many trauma programs now administer a 2 g bolus of TXA as soon as possible after the trauma, based on the results of the STAAMP trial and those of a trial involving patients with traumatic brain injury, where 2 g of TXA was safely administered prehospital by rapid infusion.9,27 Prehospital TXA is increasingly used worldwide and is associated with lower transfusion and mortality rates when introduced as a standard of care.28,29
Use of tranexamic acid for major hemorrhage. Note: IV = intravenous.
In the WOMAN (World Maternal Antifibrinolytic) trial, women with postpartum hemorrhage were randomized to receive TXA (1 g bolus, repeated at 30 min if bleeding continued) or placebo.5 Tranexamic acid reduced the risk of death from hemorrhage (RR 0.81, 95% CI 0.65–1.00), especially if given within 3 hours of start of bleeding (RR 0.69, 95% CI 0.52–0.91).
In contrast, the HALT-IT (Haemmorhage Alleviation with Tranexamic Acid — Intestinal System) trial, involving patients with substantial upper and lower gastrointestinal bleeding, found no benefit of TXA administration in reducing the risk of death from hemorrhage (RR 0.99, 95% CI 0.82–1.18) and an increased risk of venous thromboembolic complications (RR 1.85, 95% CI 1.15–2.98). In this trial, patients were randomized to receive either 4 g (1 g bolus and 3 g infusion over 24 h) of TXA or matching placebo.6 The increased risk of thromboembolic complications may have been related to the large dose used, the long duration of infusion, the older age of the patients or the inclusion of patients with cirrhosis and rebalanced hemostasis. The observed lack of benefit may have been related to the need to administer TXA shortly after the start of hemorrhage, yet many gastrointestinal bleeds are not recognized until hours after onset. The trial enrolled mostly patients with nonmassive upper gastrointestinal hemorrhage, with a wide range of time to treatment.
Tranexamic acid has been widely studied in the prevention, but not treatment of, hemorrhage among patients undergoing surgery. A systematic review identified 129 RCTs including 10 488 surgical patients.30 Tranexamic acid reduced the need for transfusion (RR 0.62, 95% CI 0.58–0.65) without increasing the risk of thromboembolic complications. Subsequently the POISE-3 (Perioperative Ischemic Evaluation–3) trial, involving 9535 randomized patients, found that 1 g of TXA at the start and end of surgery reduced the risk of major hemorrhage (RR 0.72, 95% CI 0.63–0.83) compared with placebo.8 Similarly, the ATACAS (Aspirin and Tranexamic Acid for Coronary Artery Surgery) trial, which involved 4662 patients undergoing cardiac surgery, found that TXA (50 mg/kg) reduced the risk of transfusion (37.9% v. 54.7%, p < 0.001) and need for reoperation for hemorrhage (RR 0.49, 95% CI 0.32–0.75), compared with placebo.7
What blood products, if any, should be transfused before laboratory results are available?
In patients meeting the MHP activation criteria, plasma transfusion should be started at a minimum 2-to-1 ratio of red blood cells to plasma and all other components administered based on coagulation test results. An RCT involving 680 trauma patients comparing 1-to-1-to-1 and 2-to-1-to-1 ratios of red cells to plasma to platelets found no improvement in 24-hour or 30-day mortality, critical care–free days, or hospital-free days among patients who received the 1-to-1-to-1 regimen.15
No RCTs of ratio-based resuscitation have been performed outside of trauma. An observational study of 865 massive transfusion events found no improvement in outcomes associated with high ratios compared with low ratios.31 Guidelines from the European Society of Intensive Care Medicine,32 the British Society for Haematology33 and the pan-European, multidisciplinary Task Force for Advanced Bleeding Care34 recommend initial resuscitation at a 2-to-1 ratio of red blood cells to plasma.
The fixed ratio is indicated only for the first 30–60 minutes of resuscitation and should be superseded, thereafter, by goal-directed management, guided by frequently repeated laboratory tests (including hemoglobin, platelet count, international normalized ratio [INR] and fibrinogen). This reduces unnecessary transfusions and avoids untreated coagulopathy. The platelet count does not usually drop below the transfusion threshold during MHP activation (only 40% of patients will drop below 100 × 109/L in the first 24 h).35,36
What monitoring should be undertaken to ensure appropriate targets are met?
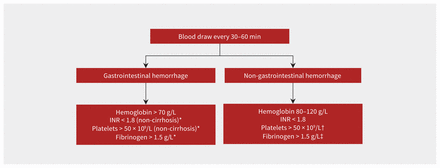
Repeated laboratory tests are required to enable goal-directed transfusion, to correct hemostatic derangements and to monitor for complications of massive transfusion. Tests for hemoglobin, platelet count, INR, fibrinogen, potassium, calcium, blood gas for pH and base excess and lactate should be performed at baseline. The guideline from the British Society for Haematology recommends testing every 30–60 minutes throughout active hemorrhage to mitigate the risk of under- and overtransfusion.33 Where possible, the results of hematological and coagulation testing, normal and abnormal, should be communicated directly to the clinical team. It is important to monitor for hypocalcemia due to citrate toxicity from blood components and for hyperkalemia from rapid infusion of red blood cells, given that storage of blood leads to high potassium levels over time. A suggested approach to laboratory targets during resuscitation is shown in Figure 3.
Frequency of laboratory testing and hemostatic targets. *No suggested target in patients with cirrhosis. †More than 100 × 109/L for patients with head injury. ‡More than 2.0 g/L for postpartum hemorrhage. Note: INR = international normalized ratio.
No evidence from clinical trials currently supports one hemoglobin threshold over another in patients with major hemorrhage. However, in patients with mostly nonmajor acute upper gastrointestinal hemorrhage, a restrictive transfusion strategy has been shown to improve outcomes. In an RCT involving 921 patients with acute upper gastrointestinal hemorrhage that compared a hemoglobin threshold of 70 g/L (post-transfusion target 70–90 g/L) with one of 90 g/L (post-transfusion target 90–110 g/L), the restrictive threshold group had lower transfusion rates and volumes (49% v. 86%, mean 1.5 v. 3.7 units, respectively), fewer rebleeding and adverse events and lower risk of death (hazard ratio 0.55, 95% CI 0.33–0.92).16 A systematic review, including 5 RCTs and a total of 1965 patients, found that a restrictive strategy improves all-cause mortality (RR 0.65, 95% CI 0.44–0.97).37 Higher hemoglobin thresholds are hypothesized to lead to higher rebleeding and mortality rates because transfusion increases portal pressure. The American College of Gastroenterology guideline recommends restricting transfusion at a 70 g/L threshold in all patients with acute upper gastrointestinal bleeding.38
Some RCTs have compared liberal thresholds to restrictive ones in surgical patients undergoing various procedures. No benefit to a liberal transfusion strategy was found, although the proportion of patients with a major hemorrhage and adherence to the trial threshold among these patients are unknown.39 A retrospective review of 418 trauma patients who were massively transfused at a single centre found the hemoglobin level 24 hours after resuscitation to be predictive of risk of death.40 Transfusion to a hemoglobin level of less than 80 g/L was associated with increased risk of death (odds ratio [OR] 3.3, 95% CI 1.6–6.7), as was transfusion to greater than 120 g/L (OR 2.5, 95% CI 1.1–5.6). Given the lack of definitive evidence, it is prudent to measure hemoglobin throughout resuscitation to avoid the consequences of under- and overtransfusion (e.g., organ hypoperfusion, anemia-related coagulopathy, fluid overload).41 The quality metric targets in Figure 3 are to maintain the hemoglobin level over 60 g/L throughout resuscitation and to minimize the proportion of patients with overtransfusion, as defined by a hemoglobin level greater than 110 g/L at 24 hours after the bleeding event.19
As with hemoglobin, no evidence from clinical trials supports one INR threshold over another to guide the transfusion of plasma. The INR is elevated in patients with major bleeding because of a multitude of factors, including coagulopathy of shock or trauma, brain injury and dilution of clotting factors by resuscitation fluids. The multidisciplinary Task Force for Advanced Bleeding Care in trauma recommends maintaining a prothrombin time of less than 1.5 times normal or monitoring for viscoelastic evidence of coagulation deficiency.34 A guideline specific to patients with cirrhosis from the European Association for the Study of the Liver (EASL) states that, given the lack of evidence to support correcting hemostatic derangements, attempts to reverse coagulopathy with plasma should not be made because of the risk of fluid overload and worsening portal hypertension.42 Lastly, the 2022 Baveno VII guideline on management of bleeding patients with portal hypertension states “transfusion of fresh frozen plasma is not recommended as it will not correct coagulopathy and may lead to volume overload and worsening of portal hypertension.”43 Given that plasma has a large volume (15 mL/kg or 1000 mL per dose), that the INR does not correlate with bleeding in patients with cirrhosis and that plasma can increase portal pressure and, hence, rebleeding risk, plasma should rarely, if ever, be employed in patients with cirrhosis. Given the lack of impact of plasma on the INR at INR levels of less than 1.8, it is reasonable to transfuse plasma to this target level in patients with major hemorrhage, no cirrhosis and an INR level greater than 1.8.44
No RCTs have compared different platelet transfusion thresholds in patients with major hemorrhage. The British Society for Haematology guideline recommends maintaining a platelet count higher than 50 × 109/L.33 The multidisciplinary Task Force for Advanced Bleeding Care in trauma recommends maintaining a platelet count higher than 50 × 109/L for all patients and higher than 100 × 109/L for patients with traumatic brain injury;34 we suggest following their guidance (Figure 3) with the exception of patients with cirrhosis, for whom platelet transfusions can be considered when bleeding is not controlled by local measures.43 Patients with major hemorrhage who are on antiplatelet agents should probably not be transfused platelets, given concern for harm or no clinical benefit, except for patients with bleeding after cardiac surgery.45–50
Trials involving patients with major hemorrhage have compared conventional laboratory testing (e.g., INR, fibrinogen) to viscoelastic testing (e.g., thromboelastography, rotational thromboelastometry) for cardiac surgery, traumatic injury and gastrointestinal hemorrhage. Viscoelastic testing is a bedside test performed on whole blood that provides guidance on the need for plasma, platelets and fibrinogen. A multicentre, step-wedge cluster randomized trial compared conventional laboratory testing to viscoelastic testing in 7402 patients undergoing cardiac surgery.51 The study found that use of viscoelastic testing reduced the risk of transfusion and major bleeding. In contrast, an RCT involving 396 patients with traumatic injury found no benefit of viscoelastic testing on patient outcomes. 13 In patients with cirrhosis and acute gastrointestinal hemorrhage (most of whom were non-massively transfused), 2 trials (n = 96 and n = 60) compared conventional laboratory assays to viscoelastic testing and found significantly lower transfusion rates with similar rates of bleeding control with viscoelastic guided transfusion. 52,53 The EASL 2022 guideline for management of bleeding in patients with cirrhosis recommends the use of viscoelastic testing. 42 Use of viscoelastic testing is increasingly common, but it is still not widely available outside tertiary care centres in Canada.54
When should fibrinogen concentrate or prothrombin complex concentrate be given?
Guidelines recommend that the fibrinogen level should be maintained above 1.5 g/L (> 2.0 g/L for patients with postpartum hemorrhage) with fibrinogen concentrate.32–34 Fibrinogen concentrate has superseded the use of cryoprecipitate in most jurisdictions in Canada, given its hemostatic equivalence and superior safety.55 No definitive RCTs have been conducted to compare higher or lower thresholds for fibrinogen replacement. The CRYOSTAT-2 RCT (NCT047004869) has completed enrolment of 1568 patients with major trauma with activation of an MHP who were randomized to receive either 15 units of cryoprecipitate (4–6 g of fibrinogen) or standard care.56 Results are expected in 2023 and will clarify whether early fibrinogen replacement reduces risk of death in trauma.
Prothrombin complex concentrate (PCC) is a small-volume, pathogen-reduced concentrate of factors II, VII, IX and X (40 mL per 1000 IU dose). The product is currently used primarily as a reversal agent for patients on warfarin with life-threatening bleeding or needing an emergency medical procedure that cannot safely be delayed 6 hours to await the effect of intravenous vitamin K. Reversal of anticoagulant therapy with PCC is not recommended in patients with gastrointestinal bleeding, except perhaps in patients with life-threatening hemorrhage. 45,46 Its use as a replacement for plasma is being investigated in several prospective RCTs (NCT03218722, NCT04534751, NCT05523297).57 The hemostatic efficacy of PCC appears similar to plasma in pilot RCTs.58,59 The use of PCC in combination with fibrinogen while awaiting transfer to a hospital with broader transfusion support capabilities is a reasonable strategy for rural hospitals that do not have the ability to prepare plasma.19,60
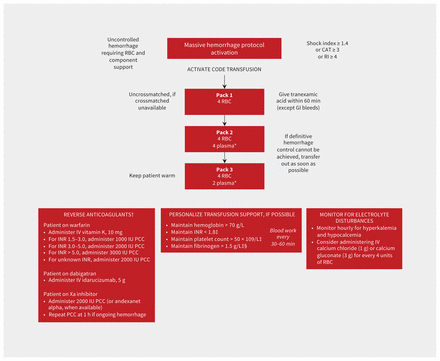
A suggested algorithm for all of the components of an MHP is depicted in Figure 4, including the reversal of anticoagulation. Further discussion on the reversal of anticoagulants in patients with a major hemorrhage can be found in recent and comprehensive guidelines.45,61 Suggested modifications for MHPs for different populations are provided in Table 2.
Algorithm for a generic massive hemorrhage protocol for patients with major bleeding. A Critical Administration Threshold (CAT) score of 3 or higher (CAT 3+) is defined as the need for transfusion of 3 or more red blood cells in the first hour of resuscitation. A Resuscitation Intensity (RI) score of 4 or higher is defined as the need for 4 or more of any of the following in the first 30 min: unit of blood (red cell, plasma or platelet), 500 mL of colloid or 1000 mL of crystalloid. The shock index is calculated as the heart rate divided by systolic blood pressure. *If plasma is unavailable in rural settings, consider 2000 IU prothrombin complex concentrate (PCC) and 4 g of fibrinogen; avoid the use of plasma in patients with cirrhosis. †Reversal is not recommended for patients with gastrointestinal (GI) bleeding unless hemorrhage is life-threatening. ‡No suggested target for patients with cirrhosis. §>2.0 g/L for postpartum hemorrhage. Note: INR = international normalized ratio, IV = intravenous, RBC = red blood cells.
Modifications to massive hemorrhage protocols according to specific circumstances
Conclusion
Major hemorrhage is life-threatening and its management is challenging, especially in rural settings where specialized blood products and laboratory tests, and source control of the bleeding, are sometimes not available. Massive hemorrhage protocols provide evidence-based guidance and can be adapted depending on the location of bleeding and patient characteristics. Rapid administration of TXA improves patient outcomes except among those with a gastrointestinal bleed. Unanswered questions to be addressed in future research are in Box 2.
Box 2:Unanswered questions
Does prehospital transfusion improve patient outcomes?
Does whole blood have a role in the early management of major hemorrhage?
What is the optimal laboratory platform (i.e., best machine) for hemostatic testing to guide transfusion decisions?
What is the role of factor concentrates in patients with major bleeding?
What are the appropriate resuscitation targets for hemoglobin, platelet count, the international normalized ratio and fibrinogen during major hemorrhage?
How should remote and rural hospitals effectively manage hemorrhaging patients while waiting to transfer patients for definitive hemorrhage control?
Footnotes
Competing interests: Jeannie Callum has received research funding from Canadian Blood Services (produces and distributes blood components) and Octapharma (manufactures prothrombin complex concentrate and fibrinogen concentrates). She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies, and on the data safety monitoring boards for the TRACE (Tranexamic Acid for Subdural Hematoma) trial, a University of Ottawa pilot study of tranexamic acid for hypoproliferative thrombocytopenia, and the FEISTY (fibrinogen replacement in trauma) trial. Alan Barkun has received reimbursement for consulting from AstraZeneca and Medtronic. Keyvan Karkouti has received research funding, consulting fees and honoraria from Octapharma, and consulting fees from Werfen (provides viscoelastic point of care testing). He sits on review boards with the Canadian Institutes of Health Research and the Heart and Stroke Foundation. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/












Podcast