Joint tuberculosis (TB) is an uncommon manifestation of TB that typically presents with subacute or chronic atraumatic inflammatory symptoms in single, large, weight-bearing joints.
In Canada, epidemiological risk factors for TB include extended time spent in countries with high burden of TB, known exposure to TB or being part of a population disproportionately affected by TB (e.g., people born outside of Canada, those of Indigenous ethnicity or those with a history of homelessness or incarceration).
Synovial thickening, joint effusions, juxta-articular osteopenia, peripheral osseous erosions and gradual joint space narrowing on magnetic resonance imaging or computed tomography are suggestive of joint TB.
Specialized mycobacterial culture is the gold standard for diagnosis of TB, but nucleic acid amplification tests are increasingly accessible and allow for rapid diagnosis of joint TB.
Treatment for joint TB should be started promptly for preservation of joint function and prevention of irreversible destruction.
A 47-year-old man presented to the emergency department with 2 weeks of acute-on-chronic pain in his right knee. He described a 5-year history of chronic pain in his right knee, with 1 flare-up 3 years before presentation, which had resolved after a few weeks of ibuprofen use. He had not previously sought care for this problem. He was otherwise healthy, with no chronic medical conditions or previous surgeries, and he tested negative for HIV. He had smoked crystal methamphetamine daily for the last 10 years and had no history of intravenous drug use. He had immigrated from the Philippines 16 years before and had not returned.
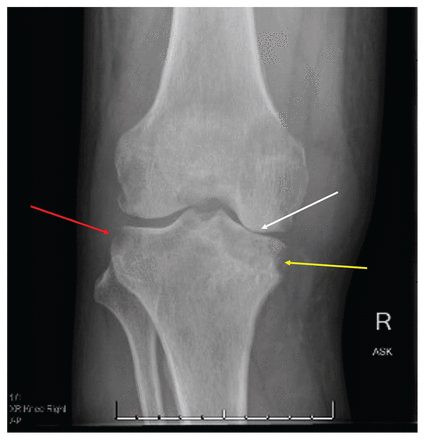
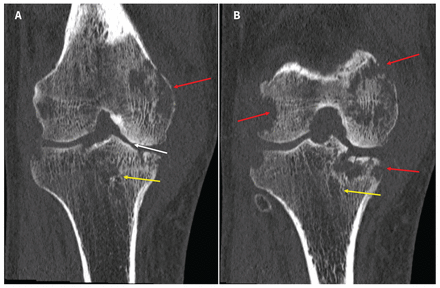
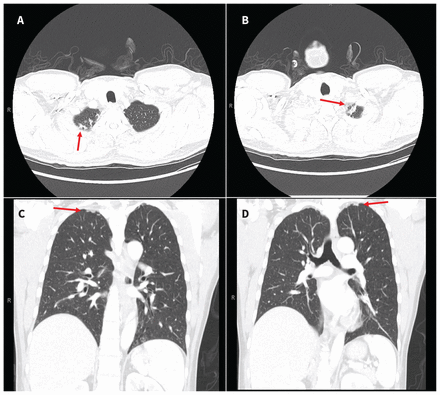
When examined, the patient was afebrile with normal vital signs. His right knee was swollen with limited flexion and extension. He did not have any overlying erythema or tenderness to palpation of the bursa or the joint line. He had no other inflamed joints. Initial laboratory results showed a leukocyte count of 8.4 (normal 4–11) × 109/L and an elevated C-reactive protein level of 34.4 (normal < 3.1) mg/L. A radiograph of the patient’s right knee showed bony destruction of the knee joint and an irregular lucency of the medial tibial plateau (Figure 1). A computed tomography (CT) scan showed cortical destruction with peripheral osseous erosions and juxta-articular osteopenia (Figure 2). The patient was admitted to the internal medicine service and the initial treating physicians were concerned that he may have had a malignant disease; they ordered a CT scan of the chest, abdomen and pelvis, which showed mild, biapical lung scarring (Figure 3).
Radiograph of the right knee joint of a 47-year-old man with joint tuberculosis, showing peripheral osseous erosions (red arrow), irregular lucency of the medial tibial plateau (yellow arrow) and joint space narrowing (white arrow).
Serial computed tomography scans (coronal view) of the right knee of a 47-year-old man with joint tuberculosis, showing the Phemister triad of tuberculosis arthropathy including juxta-articular osteopenia (yellow arrows), peripheral osseous erosions (red arrows) and joint space narrowing (white arrow, panel A).
Computed tomography scans of the lungs of a 47-year-old man with joint tuberculosis, showing mild biapical scarring (arrows), suspected to be secondary to previous tuberculosis infection. (A) and (B) are serial axial images; (C) and (D) are serial coronal images.
Bloody synovial fluid was aspirated from the patient’s right knee joint, with a leukocyte count of 36.7 × 109/L (79% neutrophils, 15% lymphocytes, 6% monomacrophages) and an erythrocyte count of 150 × 109/L, and no crystals or organisms on Gram stain. Cultures for mycobacteria were negative on acid-fast bacilli staining. Given these findings, bacterial septic arthritis was suspected and he was treated with empirical antibiotic therapy (intravenous [IV] ceftriaxone 2 g daily, and IV vancomycin with a target trough of 10–20 mg/L). After 6 days, the patient’s knee had not improved and bacterial cultures from synovial fluid remained negative. The internal medicine team consulted our infectious diseases team.
We suspected tuberculosis (TB) based on 3 factors, namely the chronic nature of the monoarthritis, the pulmonary apical scarring on chest imaging and that the patient had emigrated from the Philippines — designated as a country with a high burden of TB by the World Health Organization (WHO), with the fourth highest national incidence of TB globally.1 We identified no other identifiable risk factors for TB, such as previous incarceration, homelessness or known close contact with someone with TB.
Nucleic acid amplification testing (NAAT) for TB using the GeneXpert MTB/RIF Ultra assay (Cephied) was performed on samples of synovial fluid, which confirmed the presence of Mycobacterium tuberculosis. Smear testing of sputum samples were negative for acid-fast bacilli. We prescribed standard weight-based dosages of rifampin (10 mg/kg/d), isoniazid (5 mg/kg/d) with pyridoxine (25 mg/d), pyrazinamide (25 mg/kg/d) and ethambutol (15 mg/kg/d) and discharged him to continue this therapy under care of the provincial TB program.
Four weeks later, samples of the patient’s synovial fluid showed growth of M. tuberculosis that was susceptible to the first-line anti-TB medications he was receiving. Mycobacterial cultures from sputum samples were negative, suggesting that he did not have pulmonary TB. At follow-up after the 9-month course of treatment, the patient had considerable improvement of his knee pain (pain only with extended use), periarticular and lower leg swelling and range of motion (full flexion and extension). He had returned to work and was ambulating without need for a mobility aid. Given the extensive destruction of the knee joint, we had planned to refer him to an orthopedic surgeon for consideration of knee arthroplasty. However, he was lost to follow-up, and subsequent knee imaging and surgical referral could not be completed.
Discussion
In 2020, the incidence of TB in Canada was 4.7 per 100 000 people, consistent with the previous 10 years.2 However, people born outside of Canada and Canadian-born Indigenous peoples are disproportionately affected.2 Bone and joint TB, including both vertebral and extra-axial joint disease, is an uncommon manifestation of extrapulmonary TB. In Canada, this form of TB accounts for about 2.5% of all TB cases per year, and around half of these cases have vertebral disease.3 In low-income regions, bone and joint TB is more commonly seen among children, usually developing shortly after primary infection. In high-income countries, however, it is most commonly seen among adults, resulting from TB reactivation, as we suspected with our patient.3 Extra-axial disease usually presents as a monoarthritis that affects the knee or hip.
Diagnosis may be difficult and is often delayed by many months, particularly in countries where TB is not endemic.4,5 Key features of TB include the presence of risk factors, the long duration of symptoms and constitutional symptoms. Unlike in bacterial septic arthritis, systemic infectious symptoms such as fevers and chills, rigors or elevated heart rate are usually absent in tuberculous arthritis. Other differential diagnoses include bacterial septic arthritis, crystal arthropathy, systemic rheumatic disease (such as rheumatoid arthritis) and osteoarthritis. Poncet disease, a reactive polyarthritis associated with extra-articular TB disease, should also be considered if several joints are involved and the patient has evidence of nonerosive arthritis.6 Findings from physical examination are nonspecific and include restricted range of motion, swelling and joint effusion.7 Joint erythema, warmth and tenderness may be absent, unlike in crystal arthropathy or bacterial septic arthritis.
Typical radiologic findings in early disease show thickening of the synovium and joint effusions — similar to other causes of monoarthritis such as septic arthritis or crystal arthropathy.8 Early TB arthritis may also be misdiagnosed as osteoarthritis if imaging shows only joint space narrowing.8 Later-stage disease has features of juxta-articular osteopenia, peripheral osseous erosions and joint space narrowing, known as the Phemister triad.9 Some chronic cases may have sinus tract formation.8
Plain radiographs may show the Phemister triad in later-stage disease, but magnetic resonance imaging (MRI) and CT are the best imaging modalities for disease characterization. In particular, MRI offers the most specificity for TB arthritis, but timely availability is often limited; therefore, CT may be used as it offers more specific disease characterization than plain radiographs. 8 However, because joint TB has no pathognomonic imaging findings, particularly early in the disease course, the diagnosis cannot be made based on imaging alone and requires microbiological confirmation.
Diagnosis is typically achieved by synovial fluid analysis or synovial biopsy with microscopy for acid-fast bacilli and mycobacterial culture. However, testing synovial fluid for acid-fast bacilli has a low sensitivity of about 30% and cultures take weeks to detect growth. Mycobacterial cultures also require specialized laboratories that are certified for biosafety containment level 3 and have clinical mycobacteriology accreditation to ensure quality assurance.3 Joint fluid analysis often finds a leukocyte count in the inflammatory range, typically 10–20 × 109/L, but may be higher.7
Newer methods for molecular detection of M. tuberculosis such as NAAT are rapid, more geographically accessible and do not require specialized laboratories. The most studied and widely available NAAT assay recommended by WHO is the automated, cartridge-based GeneXpert MTB/RIF and the newer generation, GeneXpert MTB/RIF Ultra. A recent review of the diagnosis of extrapulmonary TB with the GeneXpert assay found a sensitivity of 60%–100%, depending on specimen type, and a specificity of 87.5%–100% for bone and joint specimens.10 We did not diagnose TB in our patient until 5 years after symptom onset, by which time he had considerable destruction of the knee joint. Despite the presence of joint destruction, TB arthritis typically responds well to medical therapy and is usually treated for 6–12 months. Treatment duration should be extended for patients with increased risk of relapse, such as those with extensive disease at presentation.3 Unlike other causes of septic arthritis, TB arthritis does not require acute surgical management; surgery is reserved for treatment of complications related to joint destruction after completion of TB treatment.3
Conclusion
Clinicians should consider joint TB in patients who present with subacute joint pain and swelling with joint fluid analysis suggestive of an inflammatory arthritis, and in patients who do not respond to standard therapy for inflammatory or septic arthritis. In addition to mycobacterial cultures, NAAT may aid timely diagnosis and management.
The section “Cases” presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Adrianna Gunton drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/