Abstract
Background: Since the implementation of medical assistance in dying (MAiD), deceased organ donation after MAiD has been possible in Quebec. We sought to describe organ donations after MAiD in the first 5 years after this practice was implemented in Quebec.
Methods: We reviewed all cases referred for donation after MAiD from January 2018 to December 2022. We presented all data descriptively with no comparison statistics.
Results: Transplant Québec received 245 referrals for donation after MAiD, of which 82 were retained (33.5%). Of the 163 nonretained referrals, 152 (93.2%) had a recorded reason, including 91 (55.8%) for medical unsuitability on initial screen (e.g., organ dysfunction, medical history), 34 (20.8%) for patient refusal and 21 (12.9%) instances where patients withdrew from the MAiD process entirely. Six patients died before MAiD. Eighteen of the 82 retained cases were cancelled later in the process, almost all (n = 17, 94.4%) because of medical contraindication discovered during detailed donor evaluation. Sixty-four patients became actual donors after MAiD, increasing from 8 in 2018 to 24 in 2022. The total conversion rate from referral to an actual donor was 26.1% (64/245). A total of 182 organs (116 kidneys, 20 livers and 46 lungs) were transplanted after MAiD. During the study period, MAiD donors represented 8.0% (64/803) of total deceased donors, increasing from 4.9% (8/164) in 2018 to 14.0% (24/171) in 2022.
Interpretation: These data describe a substantial increase in deceased donation after MAiD in the first 5 years of implementation in Quebec. Future studies should focus on how to optimize systems to ensure these requests are treated in the most ethical and medically effective way.
Since passage in 2015 of a provincial law allowing medical assistance in dying (MAiD), followed the next year by Canadian federal legislation allowing MAiD, Quebec and other Canadian provinces became some of a few jurisdictions in the world where deceased organ donation after MAiD is possible.1 As opposed to assisted suicide, where oral agents are provided to a patient for self-administration, in the MAiD context agents are injected intravenously and result in a predictably rapid death. Pioneering programs in Belgium and the Netherlands had already reported organ donation after MAiD,2,3 so Canadian organ donation organizations recognized the potential for organ donation after MAiD. However, programs delayed development of organ donation after MAiD to ensure implementation of the MAiD program was independent of any issues around organ donation. Ethical and logistic concerns were carefully considered during protocol development for donation after MAiD protocol in Quebec,4–6 established to be consistent with concurrently developed best practices by Canadian Blood Services and in exchange with other provincial organ donation organizations. 7 The Transplant Québec program emphasized the need for the MAiD decision to be completed before discussion of potential organ donation after MAiD and that donation decisions have no impact on access to MAiD services.
Canada’s first donations after MAiD were completed in 2016 and the first 2 donations after MAiD in Quebec were completed in late 2017 following patient-initiated request.8 Since then, a few published reports have described the outcomes of the MAiD process. A recently published international round table described national-level data from Belgium, the Netherlands and Canada.9 An earlier Canadian report also reported numbers of donors, referrals and organs transplanted.8 These reports did not include reasons why referred patients did not become actual donors, the proportion of total deceased donors represented by donation after MAiD or other details of the process. We sought to describe the outcomes of patients referred for organ donation after MAiD and the impact of those donors on the Quebec donation and transplantation system from 2018 to 2022.
Methods
Study setting
Legislation for MAiD was approved in Quebec in 2015 and federally in Canada in 2016. Eligibility criteria are defined by provincial legislation and policy, and include evaluation for presence of a severe and intractable condition and competency to consent to MAiD.10 Although criteria for MAiD have changed to include MAiD for patients who lose competency between the initial request and administration, during the study period, all patients were required to be competent up until the moment of MAiD. As of March 2021, patients were no longer required to be actively in end-of-life care to be eligible to access MAiD services.
No personnel from Transplant Québec have any role in defining MAiD eligibility or evaluating patients for MAiD eligibility. Transplant Québec has no contact with patients who are potential donors until the decision to pursue MAiD is confirmed. Before 2018, physicians were discouraged from mentioning the possibility of donation, but since 2018, physicians who provide MAiD are encouraged to discuss organ donation with eligible patients after the decision to pursue MAiD is finalized. 11 If the patient expresses interest, the physician makes a referral to Transplant Québec.
At referral, patient information is given to Transplant Québec staff, who coordinate an initial evaluation of medical eligibility. Absolute contraindications are limited only to current or remote history of neoplasm and certain infectious agents (e.g., West Nile virus). All other patients are considered for donation on a case-by-case basis, with final decisions resting with transplant programs. Relative medical contraindications are frequent and highly context dependent. Contraindications include factors related to the potential donor and the state of potential recipients on the waitlist. Relative contraindications are related to age, organ dysfunction and comorbidities such as hypertension or diabetes. These aspects do not have absolute exclusion criteria, since organs that may otherwise be rejected might be accepted if the waitlist includes a matched recipient with an acute risk of morbidity or mortality.
Assuming no medical contraindications, the coordinator at Transplant Québec explains the basics of donation after MAiD to the provider and patient. The 2 most notable changes to the MAiD process when donation is a possibility are the addition of organ eligibility testing (laboratory and imaging studies) and the fact that the MAiD process must occur at the hospital because the organs will no longer be perfused after circulatory arrest, requiring immediate organ recovery to avoid ischemic damage. These alterations are explained to the patient, who is given at least 24 hours to consider the process before being recontacted by Transplant Québec. Patients are also informed that acceptance of donation or not has no impact on access to MAiD services.
Referrals that progress past initial telephone conversations are defined as retained referrals. Patients with retained referrals are subject to further evaluation, including visits between the coordinator and the patient to confirm consent, a detailed review of the patient’s medical history, organ eligibility investigations and organ allocation to recipients.
Unlike some Canadian jurisdictions, Transplant Québec is responsible only for deceased donation of solid organs and not for tissue (e.g., corneas or heart valves). During the study period, only lungs, liver and kidneys were considered for recovery and transplantation after determination of circulatory death, whether the mechanism of death was MAiD or withdrawal of life-sustaining measures. Potential deceased donors were referred to Héma-Québec, the provincial blood and tissue bank.
If a suitable recipient is found and no contraindications are identified, the MAiD process occurs in or near the operating room, in the presence of family or friends. Organ recovery occurs almost immediately after determination of death. Death determination is done in accordance with provincial and Canadian guidance for donation after circulatory death.12
The COVID-19 pandemic presented specific challenges over the course of the study period. From March 2020 through October 2020, no donation after MAiD was performed because of the resource implications on the system and concerns around possible donor-to-recipient transmission of SARS-CoV-2. These restrictions decreased all forms of donation and transplantation in Canada and internationally,13 including donation after MAiD.
Study design
We retrospectively reviewed data from all patients referred to Transplant Québec for consideration of organ donation after MAiD in Quebec from January 2018 to December 2022, the first 5 full years where this practice was integrated into the Quebec system. During the study period, Transplant Québec staff routinely recorded any telephone referral of a patient who would potentially be eligible for organ donation after MAiD. All referrals were included and we had no exclusion criteria.
The primary outcome was the number of actual organ donors after MAiD, defined as patients from whom at least 1 organ was recovered for the purpose of transplantation. Secondary outcomes included demographics of actual donors, conversion rates of referrals to actual donors, reasons for nonprogression from a referred to actual donor, number and type of organs transplanted from actual donors and warm ischemic time, defined as time from administration of the MAiD agents to injection of organ preservation solutions.
Data sources
All data related to donors or the number of organs donated are held by Transplant Québec, the provincial organ donation organization responsible for the coordination of all deceased donation activity in the province. Organ donation coordinators at Transplant Québec input clinical and demographic information collected either through telephone interviews or on chart review into the iTransplant donor management system. This database is used to manage data and transmit information to transplant programs from all potential donors — not just potential donors after MAiD — referred to Transplant Québec. One author (M.D.L.) extracted data regarding potential and actual MAiD donors from this clinical database and transferred data to an Excel spreadsheet for analysis. Access to iTransplant is limited to a password-protected portal for Transplant Québec staff, and we stored all spreadsheets containing patient information on password-protected laptops. We retrieved data related to the number of MAiD cases performed in Quebec and MAiD policies from publicly available reports from the Quebec Commission on end-of-life care.10
Data analysis
All data are presented descriptively; we performed no comparison statistics.
Ethics approval
This project was reviewed by the research ethics board of the research centre of the CHU de Québec, Université Laval. As a retrospective, anonymized report with no comparison to other practices, the report was not deemed to require further consent from patients and was given a waiver from further evaluation by the research ethics committee.
Results
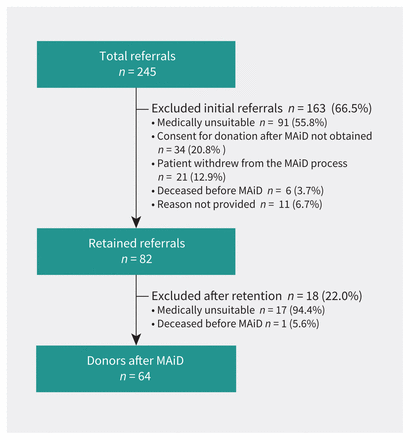
During the study period, Transplant Québec received 245 referrals for donation after MAiD. The annual number of referrals increased from 21 in 2018 to 109 in 2022. Figure 1 details the progression of referrals to actual donors or exclusion. We retained and confirmed initial consent for 82 (33.5%) of these referrals. Of the 163 nonretained referrals, the reason for refusal was documented for 152 patients (93.2%) (the remaining 11 had other or unrecorded reasons for refusal), including 91 (55.8%) patients excluded for medical unsuitability on initial screening (e.g., organ dysfunction, medical history), 34 (20.8%) patients who refused the process of donation after MAiD (e.g., refusal to receive MAiD in the hospital as opposed to at home) and 21 (12.9%) patients who withdrew from the MAiD process entirely. Six patients died before MAiD.
Referrals considered for organ donation after medical assistance in dying (MAiD).
Eighteen donations were cancelled after initial retention, almost all (n = 17, 94.4%) secondary to medical contraindication discovered during the donation evaluation. These contraindications were often found during the detailed review of the patient’s medical history by the donation coordinator or based on laboratory or imaging tests done during the organ eligibility evaluation. The remaining patient died before MAiD was administered. No patient withdrew consent during the evaluation and organization phase. Sixty-four patients became donors after MAiD, increasing from 8 in 2018 to 24 in 2022 (Table 1). The conversion rate was 26.1% (64/245) of all referrals.
Annual deceased organ donors after medical assistance in dying (MAiD) and total patients who underwent MAiD in Québec
The average age of actual donors after MAiD was 60 (range 40–76) years and most were male (n = 41, 64.1%). The most common diagnosis among donors was neurodegenerative disorders (n = 54, 84.3%), most commonly amyotrophic lateral sclerosis. The remaining diagnostic categories included terminal cardiopulmonary disorders (n < 5) or other, such as chronic pain syndromes and spinal cord injury (n = 6, 9.4%).
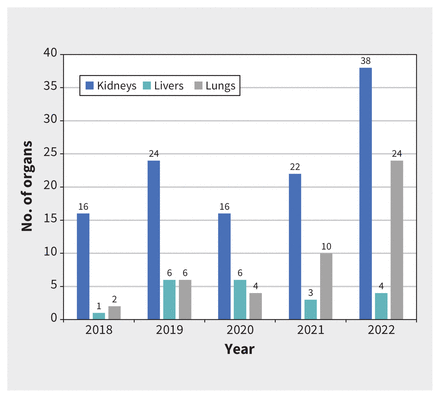
Administration of MAiD was according to the hospital and patient preferences, either on the ward, in an area proximal to the operating room, or in the operating room. The average time from administration of the MAiD agents to determination of death was 12.6 (range 3–28) minutes; the average warm ischemic time was 26.6 (range 16–43) minutes. A total of 182 organs (116 kidneys, 20 livers, 46 lungs) were transplanted for 2.9 organs used per donor. Figure 2 details the number of kidneys, livers and lungs transplanted over the study period.
Transplanted organs from donors who underwent medical assistance in dying in Quebec, 2018–2022.
The 64 actual donors after MAiD in the study period represented 8.0% of the 803 total deceased donors in the province. As detailed in Table 1, the annual percentage of deceased organ donors from MAiD as a portion of the total number of deceased organ donors (including MAiD and other pathways) increased from 4.9% (8 of 164 donors) in 2018 to 14.0% (24 of 171 donors) in 2022.
This increase in donation after MAiD occurred in the setting of an increase in use of MAiD. Quebec reports MAiD data from Apr. 1 to Mar. 31 (as opposed to calendar year, as reported by Transplant Québec), with the total growing from 968 patients in 2017–2018 to 3663 patients in 2021–2022 (Table 1).
Interpretation
We describe an increase in deceased organ donation after MAiD in the first 5 years of implementation of MAiD in Quebec. This type of organ donation represented 14% of Quebec’s total deceased donation activity in 2022.
Reports from Canada as a whole — including Quebec — indicate that, in 2021, 59 (8.0%) of 734 total deceased donors used MAiD.9,14 In 2020, the Netherlands reported 11 (4.3%) of 255 deceased donors used MAiD.9,15 Although rates of donation after MAiD in Quebec may appear higher, we caution against rate comparisons with other jurisdictions because the current report was not a comparative study and definitions could vary among jurisdictions.
Our data do not allow for detailed analysis of the factors that have contributed to the development of MAiD in Quebec or even the ultimate potential for donation after MAiD. Current protocols for MAiD evaluation do not require mandatory referral of potential donors nor audits of whether these referrals are happening. It is impossible to know how many patients are offered donation after MAiD or what the consent rate is among that group. However, based on MAiD epidemiology, it is likely that not all eligible patients were asked about organ donation. Rates of MAiD have been increasing steadily year over year in Quebec, rising from fewer than 1000 patients in 2017–2018 to more than 3500 patients in 2021–2022.10 Throughout the study period, most of those patients had metastatic cancer, but about 10% of patients had neurodegenerative or cardiopulmonary conditions as their primary diagnosis leading to a MAiD request.10 Patients with non-neoplastic diagnoses are potentially eligible for organ donation; if all of them were identified and referred, we would have expected more than the observed 109 referrals in 2022. It is possible that the patient’s MAiD provider discussed the potential for donation, but the patient was not interested in the alterations to typical MAiD that would be required, such as death in hospital or the medical work-up required. However, without a system to track whether referral of eligible patients is occurring, it is impossible to know how frequently those situations are occurring.
We analyzed reasons for nonconversion of referrals to actual donors, and these reasons comprised both modifiable and nonmodifiable factors. The most frequent reason for a patient to exit the donation pathway was the determination of medical unsuitability on review of the patient’s past and current medical status. Most of those medical exclusions were identified at the initial screening. Although those reasons are nonmodifiable, a substantial number of patients withdrew from the process over concerns about some aspect of the process of donation after MAiD. This is a potential opportunity to create processes that are better adapted to patient needs, which could lead to more patients accepting donation as part of their MAiD process.
Our findings demonstrate that the pathway for donation after MAiD results in a rapid and predictable form of donation after circulatory determination of death. The longest warm ischemic time was 43 minutes, and no donation was cancelled because of prolonged warm ischemic time. We did not analyze recipient outcomes, although reports from other centres suggest that short-term outcomes for organs recovered after MAiD are comparable to those from traditional pathways of donation after circulatory or neurological determination of death.16–18 The average number of transplanted organs per donor (2.9) is similar to that of all standard-criteria donors after circulatory death in Quebec in 2021 (2.8).19
We focused on quantitative outcomes of the donation aspects of the process. Further research is needed on both the donation and the transplantation aspects of this process. At a minimum, the rate of, and reasons for, refusal to donate by potential donors needs to be better understood. This would involve quantitative and qualitative surveys of patients, caregivers and health care providers. More information regarding the outcomes of donated organs is needed to understand graft viability compared with other donation pathways. System performance merits study to ensure that logistic barriers to donation are understood and that possible innovations (e.g., developing the possibility to initiate the MAiD process at home) are explored.20,21 As MAiD criteria change or expand, ethical analyses will be required for the evaluation of potential donors pursuing MAiD for underlying psychiatric or other conditions. Some of these issues have been addressed in the recently updated Canadian guideline on donation after MAiD.22 Future research would benefit from standardized reporting across Canada and a multidisciplinary research program designed to evaluate these inter-related issues.
Limitations
Our data were collected retrospectively and the system was evolving over the time frame of the study, which limits what can be concluded from our findings. For instance, an initial recommendation in 2016 suggested that the request for donation after MAiD must come from the patients themselves, but since June 2018, Transplant Québec has recommended that MAiD providers mention the possibility of donation to patients without metastatic cancer.11 Donation after MAiD was deeply affected by the COVID-19 pandemic, including full or partial shutdowns of activity during 2020 and 2021. Furthermore, the databases we used were not designed for research purposes, which meant that data were incomplete and lacked granularity regarding issues such as details on reasons for refusal. The data were not formally validated, although Transplant Québec is the sole agency responsible for organ donation in the province and maintains the sole database of referred potential donors.
Conclusion
Our analysis of data related to organ donation after MAiD in Quebec shows that organ donation organizations can establish systems that honour the wishes of patients pursuing MAiD to donate their organs after their death. However, much remains to be learned regarding how to optimize the system to ensure that donation requests are treated in the most ethical and medically effective way. Interactions between MAiD providers and donation specialists should emphasize the potential for donation after MAiD to ensure that all eligible patients are offered the opportunity to pursue donation. Patients considering MAiD are among the most vulnerable patients in the health care system as they have intractable diseases that cause them immense suffering. The desire of some patients to help others after their death must be honoured, but in doing so, donation professionals must assure the system respects their autonomy and dignity.
Acknowledgements
The authors would like to thank Catherine Lachance for her work in collating and organizing the Transplant Québec data. They also acknowledge the Québec-based recovery or transplant surgeons who have participated in the donation after MAiD process recovered or transplanted organs from these patients, including Dr. Marc-Antoine Despatis (CHU de Sherbrooke); Dr. Azemi Barama, Dr. Alain Duclos, Dr. Pasquale Ferraro, Dr. Michel Lallier, Dr. Jacques Malaise, Dr. Peter Metrakos, Dr. Michel Morin, Dr. Basil Nasir, Dr. Nicolas Noiseux and Dr. Louis-Mathieu Stevens (Centre Hospitalier de l’Université de Montréal); Dr. Ghislain Nourissat (CHU de Québec); Dr. David Badrudin and Dr. Gabriel Chan (L’Hôpital Maisonneuve-Rosemont); and Dr. Jeffrey Barkun, Dr. Steven Paraskevas, Dr. Jean Tchervenkov and Dr. George Zogopoulos (McGill University Health Centre). Ultimately, these data represent patients who have chosen to alter their end-of-life plans to include a truly altruistic act into their end-of-life care. We are honoured to assist them in the realization of their generosity.
Footnotes
Competing interests: Prosanto Chaudhury is a board member with the Canadian Society of Transplantation and a medical director, transplantation, with Transplant Québec. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Matthew Weiss conceived the study. All of the authors contributed to study design. Mathilde Dupras-Langlais acquired data. All of the authors contributed to data analysis and interpretation. Matthew Weiss drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Funds donated from the McGill University Health Centre, held by Prosanto Chaudhury, were used to pay publication fees.
Data sharing: No data will be shared publicly, and all data were held at Transplant Québec.
- Accepted November 8, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/