Abstract
Background: The COVID-19 pandemic is suspected to have affected cancer care and outcomes among patients in Canada. In this study, we evaluated the impact of the state of emergency period during the COVID-19 pandemic (Mar. 17 to June 15, 2020) on cancer diagnoses, stage at diagnosis and 1-year survival in Alberta.
Methods: We included new diagnoses of the 10 most prevalent cancer types from Jan. 1, 2018, to Dec. 31, 2020. We followed patients up to Dec. 31, 2021. We used interrupted time series analysis to examine the impact of the first COVID-19–related state of emergency in Alberta on the number of cancer diagnoses. We used multivariable Cox regression to compare 1-year survival of the patients who received a diagnosis during 2020 after the state of emergency with those who received a diagnosis during 2018 and 2019. We also performed stage-specific analyses.
Results: We observed significant reductions in diagnoses of breast cancer (incidence rate ratio [IRR] 0.67, 95% confidence interval [CI] 0.59–0.76), prostate cancer (IRR 0.64, 95% CI 0.56–0.73) and colorectal cancer (IRR 0.64, 95% CI 0.56– 0.74) and melanoma (IRR 0.57, 95% CI 0.47–0.69) during the state of emergency period compared with the period before it. These decreases largely occurred among early-stage rather than late-stage diagnoses. Patients who received a diagnosis of colorectal cancer, non-Hodgkin lymphoma and uterine cancer in 2020 had lower 1-year survival than those diagnosed in 2018; no other cancer sites had lower survival.
Interpretation: The results from our analyses suggest that health care disruptions during the COVID-19 pandemic in Alberta considerably affected cancer outcomes. Given that the largest impact was observed among early-stage cancers and those with organized screening programs, additional system capacity may be needed to mitigate future impact.
The COVID-19 pandemic started in Canada in January 2020, followed shortly by various interventions to reduce strain on the health care system. These protections varied by province and territory but generally involved minimizing in-person care for health care services not related to COVID-19 and equipping nonemergency hospital units for pandemic response.1 It is possible that these protocols had far-reaching effects on the diagnosis, treatment and prognosis of diseases other than COVID-19, such as cancer, but the extent of these consequences is not yet clear.
Previous studies from several countries have reported decreased diagnoses of some cancers and changes in treatment services and timelines compared with prepandemic years.2–4 This apparent drop in cancer diagnoses indicates that patients with cancer may have delays in screening and detection owing to decreased interaction with the medical system during protections related to COVID-19. Any shift to a higher proportion of patients who receive diagnoses of later-stage cancers will have serious implications for survival outcomes.
The province of Alberta, Canada, declared a state of public health emergency on Mar. 17, 2020, and continued until June 15, 2020. Although cancer care, such as active chemotherapy and emergency surgery, was prioritized in the early stages of the pandemic, some cancer care services — including nonemergency surgeries and screening programs for breast, cervical and colorectal cancers — were delayed or paused.5 The first wave of COVID-19 reached its peak in Alberta on Apr. 30, 2020, and active cases started to decrease. Some nonurgent medical services were resumed as of early May, and by June 12, 2020, the Government of Alberta had lifted some restrictions in response to the pandemic.6
In this study, we evaluated the impact of COVID-19 on the number of cancer diagnoses in Alberta and whether there was a shift toward higher stage at diagnosis during the COVID-19 state of emergency period compared with the months before the pandemic was declared. Additionally, we assessed whether there was a difference in survival among patients who received a cancer diagnosis during the first 8 months of COVID-19 protections compared with diagnoses before the COVID-19 pandemic.
Methods
Data sources
We used the Alberta Cancer Registry to identify new cancer diagnoses from Jan. 1, 2018, to Dec. 31, 2020. This population-based registry captures information on all individuals who receive a cancer diagnosis in the province; it has maintained Gold Certification status since 2005 from the North American Association of Central Cancer Registries, based on the completeness of data, timely reporting and other data quality measures. Health information professionals complete cancer registrations through the classification of cancer type to align with national and international cancer coding and staging rules. The Alberta Cancer Registry collects demographic information (including age, sex, date of birth), tumour information (date and method of diagnosis, type and morphology, stage of cancer captured from pathology, physician and laboratory reports and electronic medical records) and mortality information (date and cause of death captured via Vital Status). In this study, we further linked patients with cancer to other databases of the provincial health care system, including any doctor visits, pharmacy dispensations, hospital admissions or ambulatory care encounters. Patients without a death outcome from Vital Status were censored at the date of last contact with any provincial health care database. Follow-up data were available up to Dec. 31, 2021, for this study. We included 10 cancer sites that were either most diagnosed or associated with screening programs in Alberta; i.e., prostate, colorectal, breast (female), bladder, lung, skin melanoma, non-Hodgkin lymphoma, cervical, uterine and kidney. We excluded stage 0 cancers, except for stage 0 bladder cancer, which was included because, since 2012, reporting in situ bladder cancer diagnoses has become common practice across Canada.7 Bladder cancer diagnosed in situ is likely to recur after treatment and has an unfavourable prognosis,8 so changes in bladder cancer incidence at this stage are important. For cancers of the same site diagnosed at different dates for a patient, we kept only the first diagnosis. For multiple cancers diagnosed on the same date for a patient, either same or different sites, the one with the most advanced TNM staging was included. We obtained quarterly population counts for the study period from Statistics Canada9 and estimated monthly populations with linear interpolation.
Statistical analysis
We used an interrupted time series design to study the impact of the COVID-19 pandemic on cancer diagnoses in Alberta.10 The unit of analysis was monthly (midmonth to midmonth) aggregate counts of cancer diagnoses of the major cancer sites, which we divided into the “pre-COVID-19” time period (Jan. 16, 2018, to Mar. 15, 2020), the “state of emergency” (SOE) period (Mar. 16 to June 15, 2020), and the “postSOE” time period (June 16 to Dec. 15, 2020). We used a Poisson regression model for our interrupted time series analysis. We calculated the following for each cancer site:
in which Cancer diagnosest is the dependent variable of monthly cancer diagnoses at time t. Populationt is the estimated population in Alberta at time t. “Time” is a continuous variable that indicates the time elapsed since Jan. 16, 2018, in the unit of month (up to Dec. 15, 2020). β1 represents the general trend in cancer incidence rates over each month. “Monthtj” is an indicator variable showing that Montht belongs to the jth of the 12 months, to reflect the seasonal changes in cancer diagnosis. β2j is a set of 11 coefficients in contrast to January as the referent month. SOEt is a dummy variable indicating the prepandemic period (= 0) or after the onset of the state of emergency in Alberta (= 1); β3 indicates the average proportional decrease in cancer diagnosis during the 3 months of the state of emergency. PostSOEt is a continuous variable indicating the number of months passed since the end of the state of emergency (June 15, 2020) and is 0 for the pre-COVID-19 and state of emergency periods. It is similar to an interaction term between “Time” and “SOEt”. β4 represents the monthly rate of how the cancer diagnoses recovered after the state of emergency. A schematic drawing of the model is shown in Appendix 1, Supplementary Figure 1 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content). We evaluated model fit with the deviance statistic and the Pearson statistic for lack-of-fit and overdispersion. For breast, colorectal, kidney and prostate cancers, where there was evidence of overdispersion, we used a negative-binomial model instead.
We defined the number of missed cancer diagnoses as the difference between the sum of the expected values of cancer diagnoses from Mar. 16 to Dec. 15, 2020, and the counterfactual cancer diagnoses had the pandemic not occurred under the same model (by setting both “SOEt” and “PostSOEt” to 0 for all t). We used 2000 bootstraps of the original data set to estimate the confidence interval (CI) of the number of missed cancer diagnoses. For each iteration, we estimated the overall differences between the expected cancer diagnoses and the counterfactual cancer diagnoses during Mar. 16 to Dec. 15, 2020. We used the 2.5 and 97.5 percentiles of the 2000 bootstraps as the limits of the 95% CI.
We used ordinal logistic regression to model whether the cancer stage distributions were different before and after the pandemic. We excluded unstaged diagnoses under the missing-completely-at-random assumption. We performed supplementary analyses on dichotomized cancer staging (stage III and IV versus stage I and II; stage IV versus stage I, II and III) with logistic regression.
We compared 1-year overall survival for 3 cohorts of patients: patients who received a diagnosis between (1) Jan. 16, 2018, and Mar. 15, 2019; (2) Mar. 16, 2019, and Mar. 15, 2020; and (3) Mar. 16 and Dec. 15, 2020. The first cohort (referred to as the “2018 cohort”) was not affected by the pandemic for diagnosis or 1-year follow-up. The second cohort (“2019 cohort”) was not affected for diagnosis but the 1-year follow-up could have been affected, whereas the third cohort (“2020 cohort”) had both diagnosis and 1-year follow-up affected. We excluded patients who received multiple primary cancer diagnoses during Mar. 16, 2018, to Dec. 15, 2020, from the analysis. We plotted Kaplan–Meier survival curves for each cancer site. We performed multivariable Cox regression with the 3 patient cohorts, adjusted for age, sex (not controlled for in breast, cervical, uterine or prostate cancers), location of residence and stage.
We performed all statistical analyses in R (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria).
Ethics approval
This study was approved by the Health Research Ethics Board of Alberta Cancer Committee (HREBA.CC-17-0034 and 19-0048).
Results
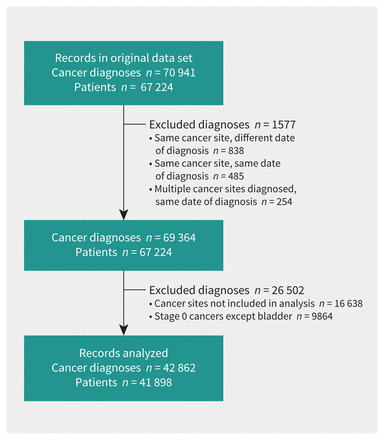
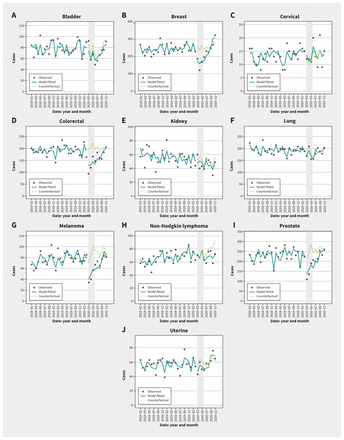
A total of 42 862 cancer diagnoses were included in our analysis after excluded diagnoses (Figure 1). Primary cancer diagnoses between January 2018 and December 2020 are shown in Figure 2. Numbers of cancer diagnoses by site and stage before, during and after the COVID-19 state of emergency in Alberta are shown in Appendix 2, Supplementary Table 1 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content).
Flow diagram of the number of patients and cancer diagnoses in Alberta between January 2018 and December 2020 included in the study. Stage 0 bladder cancer diagnoses were included.
Observed, model-fitted or counterfactual number of monthly diagnoses by cancer site in Alberta, 2018–2020. Note: state of emergency period shaded.
Breast, colorectal and prostate cancers and melanoma all had substantial reductions in diagnoses during the state of emergency compared with the incidence that would have been predicted during that time. We estimated a 33% decrease in the breast cancer incidence during the state of emergency (incidence rate ratio [IRR] 0.67, 95% CI 0.59–0.76). After the state of emergency, the monthly diagnoses recovered at a 10% rate per month (IRR 1.10, 95% CI 1.06–1.13) (Table 1). For melanoma, colorectal and prostate cancers, new diagnoses were 43%, 36% and 36% lower during the state of emergency than before it, respectively. The monthly recovery rates after the state of emergency were 9%, 8% and 8%, respectively. Bladder, kidney, lung, cervical and uterine cancers and non-Hodgkin lymphoma did not have substantial decreases during the state of emergency, or significant differences after it. The reduction in cancer diagnoses resulted in missed cancer cases when we considered a counterfactual model where the state of emergency had not occurred (Table 2). In this model, an additional 350 breast cancers, 398 colorectal cancers, 484 prostate cancers and 223 melanomas would have been diagnosed between March and December 2020, had the pandemic not occurred.
Incidence rate ratios of 10 cancers in Alberta (all stages combined) during state of emergency, compared with the incidence rate before the COVID-19 pandemic, and the monthly recovery rate after the state of emergency
Missed cancer diagnoses during March to December 2020, modelled as the difference between the counterfactual cancer diagnoses had the COVID-19 pandemic not occurred, and the modelled diagnoses
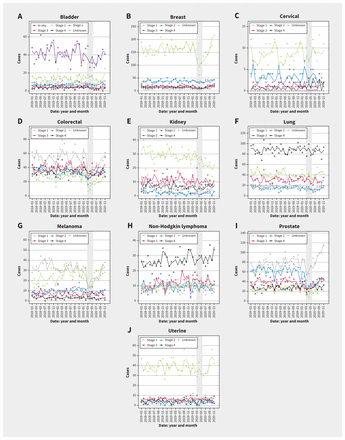
To examine whether the decreases in cancer diagnoses differed by stage during the state of emergency, we conducted an analysis comparing stage-specific relative decrease in diagnoses during the state of emergency and the recovery afterward; see Appendix 3, Supplementary Table 2 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content). In general, a larger decrease in cancer diagnoses during the state of emergency was associated with a faster recovery rate after the state of emergency for different stages of cancer. The state of emergency led to decreases in detection of stage I to IV breast cancers, which was statistically significant in stage I and III (Figure 3 and Appendix 3, Supplementary Table 2). The decrease in diagnosis of breast cancers of all stages was followed by a demonstrable recovery in detection after the state of emergency. Colorectal cancer diagnoses showed the largest decreases in diagnosis of stage I (52%) and unstaged cancers (52%), followed by a quick recovery (12% and 14% monthly rate). Similarly, melanoma had a large reduction in stage I (53%) and unstaged (49%) disease diagnoses during the state of emergency, whereas modest changes in diagnoses were observed for the stage II–IV disease, without statistical significance. There were large reductions in all stages of prostate cancer during the state of emergency, except for stage IV disease, for which we observed no change in diagnoses. Bladder, kidney, lung and uterine cancers did not show significant differences in stage-specific diagnoses during the state of emergency. To examine whether there was a shift in the distribution toward more advanced stages of cancer diagnoses after the onset of COVID-19, we carried out ordinal logistic regression as well as logistic regression on dichotomized cancer staging (III and IV versus I and II, IV versus I, II and III). A higher proportion of cancers diagnosed at more advanced stages was observed only for prostate cancer after the onset of the COVID-19 pandemic, as shown in Appendix 4, Supplementary Table 3 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content).
Observed and model-fitted number of monthly diagnoses by cancer site and stage, Alberta, 2018–2020. Note: state of emergency period shaded.
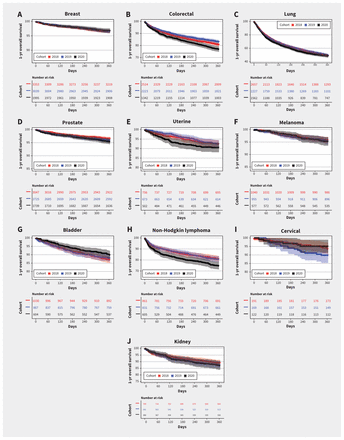
Patients who received a diagnosis of colorectal cancer and non-Hodgkin lymphoma during March to December of 2020 had a poorer 1-year survival than those who received the diagnosis in 2018, according to a log-rank test (Figure 4 and Appendix 5, Supplementary Table 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content). No other major cancer sites showed a statistically different 1-year survival between the 2020 and 2018 patient cohorts. After adjusting for age, sex, urban or rural residence, and cancer staging, we observed an increased risk of mortality for patients with colorectal cancer (hazard ratio [HR] 1.21, 95% CI 1.05–1.40), non-Hodgkin lymphoma (HR 1.43, 95% CI 1.14–1.79) and uterine cancer (HR 1.57, 95% CI 1.06–2.33) in 2020 (Appendix 6, Supplementary Table 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content).
Kaplan–Meier curve of 1-year overall survival of patients with cancers diagnosed during January 2018 and December 2020. Note: Crosses indicate censoring.
Interpretation
Our results show that the pandemic-related state of emergency in Alberta corresponded with decreased diagnoses of breast, colorectal and prostate cancers, and melanoma. According to our model, 1455 diagnoses of these 4 cancers could have been missed in 2020. The decrease in diagnoses during the state of emergency was more evident in early-stage cancers (especially stage I) than stage IV cancers. Unstaged melanoma, colorectal and prostate cancers also showed a significant decrease. Interestingly, these 3 cancers also had the highest proportions of unstaged cancers in Alberta (Appendix 2, Supplementary Table 1). The decrease in diagnoses in these 4 cancers was compensated by a monthly recovery rate ranging from 8% to 10%. By December 2020, the number of diagnoses had returned to the expected level (Figure 2), suggesting resilience of cancer care services in Alberta.
Our findings of decreased cancer diagnoses are similar to those described in other populations. Studies from the United States, the United Kingdom, the Netherlands, Germany, Japan and Canada have reported decreased incidence of cancer in the period after the implementation of protocols related to COVID-19.2,11–13 Three Canadian provinces have reported on changes in cancer diagnoses during the pandemic. In Ontario, there was a 34% reduction in incident cancers in April 2020, and Manitoba found a 23% reduction in cases in a similar period.14,15 In Quebec, there was an estimated 15% reduction during the first year of the pandemic (April 2020 to March 2021) compared with prepandemic years.16
Our findings that early-stage breast and colorectal cancer had the largest decrease in diagnoses suggest that a reduction in screening services during the first wave of pandemic-related restrictions in Alberta resulted in asymptomatic individuals receiving a diagnosis later than they would have otherwise. These results highlight the importance of screening services in reducing late-stage cancer diagnoses. A decrease or complete pause in cancer screening services occurred in other Canadian provinces during this time. In Ontario, cancer screening services decreased by as much as 100% between April and June 2020, with the largest reductions observed for mammograms among those at average risk.4 Diagnoses of cervical cancer were not significantly affected despite having a screening program, owing to the low incidence of disease in Alberta. The impact of screening program closures on cancer diagnoses in Alberta is further demonstrated by the finding that diagnoses of bladder, kidney and lung cancer did not decrease during the state of emergency. Although diagnostic services were disrupted to some degree in the early stages of the state of emergency, many services were available that could have diagnosed these cancers by other means (i.e., computed tomography scans and cystoscopies).
In our analysis of mortality changes during the state of emergency, we found increased mortality for colorectal cancer, non-Hodgkin lymphoma and uterine cancer. These findings are preliminary, and it is too soon to see whether the state of emergency will have a long-term impact on cancer mortality. Future studies should continue to monitor for trends, as delays in screening and treatment may have an effect on survival. For those who received a diagnosis of cancer requiring surgery or chemotherapy, these services were often delayed owing to demand for care related to COVID-19 in hospitals.15 According to a recent meta-analysis, each 4-week delay in surgery for common cancers like breast and colorectal cancer could result in a 6%–8% increased risk of mortality.17 This increased risk may lead to significant excess deaths in the future, even as strains on the health care system subside. After the rapid changes in incidence and early-stage diagnoses in the early stages of health care restrictions related to COVID-19, these measures returned to previous levels within months after the removal of the restrictions. However, the effect of the pandemic restrictions created a backlog that could not be cleared by merely returning to previous levels; treatment and diagnostic capacity would need to increase by at least 10% over prepandemic levels to avoid the excess mortality described previously.18
The indirect effects of the COVID-19 pandemic should continue to be studied into the future. Specific attention should be paid to the kinds of cancer treatments that have been delayed during the pandemic and whether these delays have affected some cancers more than others.
Finally, although our survival analysis extends only to 1 year, it and other studies indicate a clear impact of the pandemic on some cancer outcomes, particularly among those with early-stage cancers. Longer follow-up is needed to observe the long-term effects of the pandemic on cancer survival. Future studies should track survival among those with cancer diagnoses in 2020 compared with earlier cohorts.
Limitations
Our analyses are limited by a specific time frame and must be interpreted with caution. The first wave of the COVID-19 pandemic (beginning March 2020 in Alberta) saw the most stringent health care restrictions, including the near-total pause in cancer screening services. Future studies should examine later waves of the pandemic, in which there was more access to effective health care services despite higher levels of hospital admissions for COVID-19.
Our interrupted time series model required several assumptions: that if the pandemic had not occurred, the monthly cancer diagnoses in Alberta for 2018–2020 would have followed a log-linear trend, with seasonal variations; and that during the state of emergency, there was a proportional decrease in the number of cancer diagnoses. The proportion may not be the same over the 3 months of the state of emergency, but we estimated the average proportional decrease during that time. The final assumption is that after the state of emergency ended, the recovery in the number of cancer diagnoses would be gradual by a log-linear trend within 6 months. Our rationale was that most health care services in Alberta were still functioning on a reduced capacity after the state of emergency. Other models with different assumptions could be used to explore the effect of the state of emergency on cancer diagnosis. For example, we determined the 3-month span of the state of emergency a priori. However, some level of nonurgent medical services started to resume in early May 2020 in Alberta. Therefore, an alternative model with 2 months of state of emergency and 7 months of recovery after that period could be valid as well. The results of this alternative model are presented in the supplementary materials (Appendix 7, Supplementary Tables 6 and 7, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content). The estimated proportional decrease, monthly recovery rates and missed cancer diagnoses were similar to the main model.
The observation of increased mortality for colorectal cancer, non-Hodgkin lymphoma and uterine cancer in 2020 provides only weak evidence of a link between restrictions related to the pandemic and increased cancer mortality. The observed changes in 1-year mortality may be susceptible to year-to-year variations that are unrelated to the pandemic.
We censored the patients at their last contact with any provincial health care database, which may lead to early censoring and inflated mortality rate. We carried out a sensitivity analysis assuming that all patients had a full year follow-up until death occurs (Appendix 8, Supplementary Tables 8 and 9, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221512/tab-related-content). The 1-year survival probability and HRs were similar to the main model.
Finally, our assessment of statistical significance did not account for the large number of statistical comparisons, which could have introduced type I errors.
Conclusion
The sweeping and unprecedented measures enacted at the beginning of the COVID-19 pandemic in Alberta had an inevitable impact on cancer care. Even though treatment and urgent surgeries for cancers were prioritized when other procedures were delayed or cancelled, preventive and diagnostic services were greatly reduced. As a result, in this study, we observed a significant decrease in breast, colorectal and prostate cancers, and melanoma, with shifts toward higher stages at diagnosis, and suggestions of reduced 1-year survival of patients with colorectal cancer, non-Hodgkin lymphoma and uterine cancer. In the coming years, cancer care will likely need to adjust and operate at higher capacity to reduce any far-reaching impact on cancer outcomes.
Footnotes
Competing interests: Daniel Heng reports receiving consulting fees from Bristol Myers Squibb, Merck, Ipsen, Exelixis, Novartis, Pfizer and Eisai. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Darren Brenner, Winson Cheung and Yibing Ruan contributed to the conception and design of the work. Winson Cheung, Yibing Ruan, Devon Boyne, Tamer Jarada, Daniel Heng, Dylan O’Sullivan and Darren Brenner contributed to the analysis and interpretation of data. Emily Heer and Yibing Ruan drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Emily Heer and Ruan contributed equally.
Funding: The study was supported by the Armstrong Investigatorship in Molecular Cancer Epidemiology at the Cumming School of Medicine.
Data sharing: Data may be accessed through Alberta Health Services with appropriate data applications and ethics approval.
- Accepted May 17, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/