Abstract
Background: Therapeutic options for intermediate- or high-risk pulmonary embolism (PE) include anticoagulation, systemic thrombolysis and catheter-directed thrombolysis (CDT); however, the role of CDT remains controversial. We sought to compare the efficacy and safety of CDT with other therapeutic options using network meta-analysis.
Methods: We searched PubMed (MEDLINE), Embase, ClinicalTrials.gov and Cochrane Library from inception to Oct. 18, 2022. We included randomized controlled trials and observational studies that compared therapeutic options for PE, including anticoagulation, systemic thrombolysis and CDT among patients with intermediate- or high-risk PE. The efficacy outcome was in-hospital death. Safety outcomes included major bleeding, intracerebral hemorrhage and minor bleeding.
Results: We included data from 44 studies, representing 20 006 patients. Compared with systemic thrombolysis, CDT was associated with a decreased risk of death (odd ratio [OR] 0.43, 95% confidence interval [CI] 0.32–0.57), intracerebral hemorrhage (OR 0.44, 95% CI 0.29–0.64), major bleeding (OR 0.61, 95% CI 0.53–0.70) and blood transfusion (OR 0.46, 95% CI 0.28–0.77). However, no difference in minor bleeding was observed between the 2 therapeutic options (OR 1.11, 95% CI 0.66–1.87). Compared with anticoagulation, CDT was also associated with decreased risk of death (OR 0.36, 95% CI 0.25–0.52), with no increased risk of intracerebral hemorrhage (OR 1.33, 95% CI 0.63–2.79) or major bleeding (OR 1.24, 95% CI 0.88–1.75).
Interpretation: With moderate certainty of evidence, the risk of death and major bleeding complications was lower with CDT than with systemic thrombolysis. Compared with anticoagulation, CDT was associated with a probable lower risk of death and a similar risk of intracerebral hemorrhage, with moderate certainty of evidence. Although these findings are largely based on observational data, CDT may be considered as a first-line therapy in patients with intermediate- or high-risk PE.
Protocol registration: PROSPERO — CRD42020182163
Pulmonary embolism (PE) is the third leading cause of death from cardiovascular disease after myocardial infarction and stroke. The annual incidence of PE is 39–115 per 100 000 population.1
In Canada, the annual age-standardized mortality rate of PE is about 2.6 deaths per 100 000 population.2 An early diagnosis is essential as about one-third of deaths occur suddenly or within a few hours of the acute event, about 40% of deaths are diagnosed post mortem, and only 7% of early deaths occur in patients who were properly diagnosed and treated.3
A risk-adjusted management strategy is essential for patients with diagnoses of PE. The American Heart Association and the European Society of Cardiology (ESC) categorize PE into 3 risk categories. Patients with high-risk or massive PE include those who show hemodynamic instability. Intermediate-risk or submassive PE includes hemodynamically stable patients with a PE Severity Index (PESI) of class III–IV or simplified PESI score of 1 or higher and either elevated cardiac biomarkers or right ventricle dysfunction. Patients with low-risk PE have a PESI class I–II or simplified PESI score less than 1.4,5
The 3 major therapeutic strategies for acute PE are hemodynamic and respiratory support, anticoagulation and reperfusion therapy with either systemic thrombolysis or catheter-directed therapy. Percutaneous catheter-directed thrombolysis (CDT) allows slow and local infusion of thrombolytic material in low doses (about a quarter of the systemically administered dose), directly to the pulmonary arteries, with or without the use of mechanical or ultrasound fragmentation of the thrombus in situ.5–8
The evidence that currently supports the efficacy and safety of CDT in patients with acute PE is suboptimal. Individual studies have produced mixed results, and study limitations make it difficult to draw definite conclusions.9,10 As a result of this uncertainty, treatment guidelines have varied considerably. According to the ESC clinical consensus statement from 2022, CDT should be considered in patients with high-risk PE when thrombolysis has failed or is contraindicated. In stable patients who have failed anticoagulant therapy, CDT may be a viable option.11 The American Society of Hematology 2020 guideline panel suggested using systemic thrombolysis rather than CDT in patients with PE in whom thrombolysis is considered appropriate.12 A similar approach was taken by the CHEST Guideline and Expert Panel Report.13
The aim of this network meta-analysis was, therefore, to compare the efficacy and safety of different therapeutic strategies (anticoagulation, systemic thrombolysis and CDT) to determine the best strategy for patients with intermediate- or high-risk PE.
Methods
Search strategy
We searched for relevant clinical studies in 4 electronic databases, namely Embase, ClinicalTrials.gov, The Cochrane Library and PubMed (MEDLINE). The syntax used in database searches is in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220960/tab-related-content. The search strategies incorporated index terms, Medical Subject Headings (MeSH) and text words for the search concepts (Appendix 1). We did not include any language or date restrictions.
We included randomized controlled trials (RCTs), cohort studies and case–control studies that compared at least 2 therapeutic options, including anticoagulation, systemic thrombolysis or CDT (local thrombolysis or ultrasound-assisted CDT). We excluded duplicate reports, case reports, case series, pharmacokinetic studies in healthy adults, reviews, expert opinions, editorials, letters to the editor and comments. We included only studies involving participants with intermediate-(submassive) or high-risk PE. For this purpose, we used the authors’ definitions in each article. When no explicit definition was provided, we determined participants’ risk category according to ESC and American Heart Association guidelines (Table 1).14–16
Classification of pulmonary embolism according to European Society of Cardiology and American Heart Association guidelines
We performed the systematic review and network meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement for network meta-analyses.17 The planned analysis was registered at the PROSPERO International Prospective Register of Systematic Reviews (CRD42020182163).
Data extraction and quality assessment
One reviewer (S.Y.) identified the studies. Two reviewers independently examined the articles for eligibility (S.Y. and R.Z.). They resolved disagreements by consensus or by referring to a third reviewer (B.H.R.). We manually searched reference lists to identify additional reports. S.Y. and R.Z. extracted data independently. When needed, the reviewers contacted the principal investigators for further data extraction regarding the risk categorization of the pulmonary emboli.
We assessed the quality of observational studies using the Newcastle–Ottawa Scale.18 We used the Cochrane tool for assessing risk of bias for RCTs, and excluded those at high risk of bias.19 We also excluded observational studies with low scores (< 7) on the Newcastle–Ottawa Scale scale. We excluded studies that showed significant differences between participant groups in terms of PE severity and risk factors. We considered the following potential risk factors in our decision: age (differences in age > 4 yr among patients older than 60 yr) heart rate greater than 110 beats per minute, systolic blood pressure less than 100 mm Hg or greater than 160 mm Hg, arterial oxygen saturation less than 90%, bilirubin level twice as high as normal, other liver enzymes (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) 3 times higher than normal, cancer, liver disease, cirrhosis, stroke history, previous major bleeding, dementia and surgical history.
The primary outcomes were all-cause, in-hospital death, intracerebral hemorrhage and major bleeding. If an event was not specified as occurring in hospital, we considered the earliest reported event up to 30 days after the PE. Secondary outcomes were other bleeding events including minor bleeding, gastrointestinal bleeding and need for blood transfusion. We determined bleeding classification according to definitions in the individual studies (Appendix 1, Table S5).
Data analysis
We conducted data analysis and generated network graphs using frequentist network meta-analysis, applying a random effect model (package netmeta, version 2.7–0) as described by Rücker20 and Rücker and Schwarzer.21 We performed a design-based decomposition of Cochran Q to evaluate the homogeneity of the whole network, the homogeneity within designs and the homogeneity and consistency between designs (decomp.design function in the netmeta package).22 We considered p values less than 0.05 to be evidence of inconsistency. Forest plots also included heterogeneity measures, I2 values and lower and upper confidence limits based on the Cochran Q statistic. We also tested for local inconsistency in the network meta-analysis and determined the contribution of direct and indirect evidence (netsplit function in the netmeta package). We assessed potential publication bias with a comparison-adjusted funnel plot (funnel.netmeta function in the netmeta package).23 A nonsignificant p value indicated a symmetric funnel plot using the Egger test.24 We conducted pairwise meta-analyses for all main and secondary outcomes with direct evidence (netpairwise function in the netmeta package). We ranked treatments based on P-scores.21 We employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for network meta-analysis to assess the certainty of the results.25 We assigned a certainty rating (high, moderate, low or very low) to each comparison and outcome based on bias, inconsistency, indirectness, imprecision and publication bias.
Sensitivity and subgroup analysis
We performed a subgroup analysis for patients with intermediateto high-risk PE. In addition, we conducted sensitivity analyses without studies in which PE severity was classified as unclear; according to the type of study (RCTs v. observational studies); focusing only on in-hospital deaths (i.e., excluding deaths up to 30 days after PE that were not specified as occurring in hospital); and incorporating the articles that were excluded because of a high risk of bias.
Ethics approval
Approval by an ethics review board was not required because this study was a review and meta-analysis.
Results
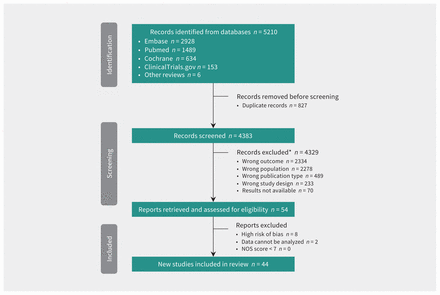
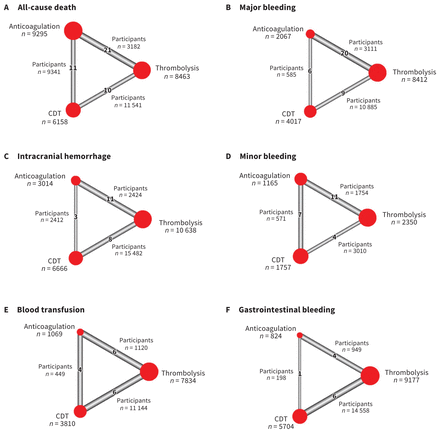
The initial search included publications up to April 2020; we updated our search in February and October 2022 using the same syntax. According to the data extraction protocol, we included a total of 44 studies, including 19 RCTs in the network meta-analysis. All studies were published and full reports were available. Results of assessment of bias and reasons for exclusion are found in Appendix 1 (Figure S10 and Table S3). Table 2 summarizes the characteristics of the included studies. The network meta-analysis diagram is shown in Figure 1. Overall, 20 006 patients were included in the network meta-analysis. A graphic representation of the number of patients in each arm and between arms is shown in Figure 2. Appendix 1, Table S2 summarizes the characteristics of the 3 treatment comparisons.
Study characteristics
Flow chart. *Studies could be excluded for more than 1 reason. Note: NOS = Newcastle–Ottawa Scale.
Net graphs of primary and secondary outcomes, showing the number of participants included in analyses of (A) all-cause death, (B) major bleeding, (C) intracranial hemorrhage, (D) minor bleeding, (E) blood transfusion and (F) gastrointestinal bleeding. Note: CDT = catheter-directed thrombolysis. The size of the red circle and corresponding sample size indicates the number of participants who received that treatment. The number of participants along the triangle sides indicates those involved in comparison of treatment arms. The thickness of the sides in the triangle indicates how many studies were conducted between treatments. As the number of articles comparing treatments increases, the thickness increases. Some studies included 3 treatment arms.
All-cause mortality
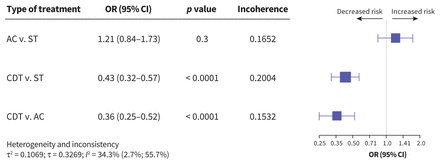
We evaluated all-cause deaths based on data extracted from 38 studies (Appendix 1, Figures S21 and S28). The overall proportion of deaths among 23 916 patients was 10.1%, and the risk of death was associated with treatment approach (Figure 3). Compared with systemic thrombolysis, CDT was associated with decreased risk of death (odds ratio [OR] 0.43, 95% confidence interval [CI] 0.32–0.57, I2 = 34.3%, moderate certainty of evidence). Compared with anticoagulation, CDT was also associated with decreased risk of death (OR 0.36, 95% CI 0.25–0.52, I2 = 34.3%, moderate certainty of evidence).
Network meta-analysis of the association between treatment for pulmonary embolism and all-cause death. Size of squares is proportional to the weight of each arm. Decreased or increased risk of the outcome is of the first type of treatment in comparison, relative to the second type of treatment. The p value indicates the probability of observing the differences between direct and indirect treatment effects. The presence of incoherence is indicated by a p value less than 0.05. Note: AC = anticoagulation, CDT = catheter-directed thrombolysis, CI = confidence interval, OR = odds ratio, ST = systemic thrombolysis.
Major bleeding
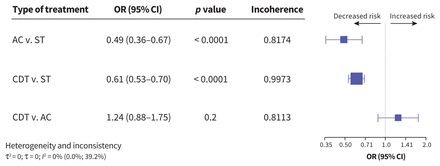
Major bleeding was assessed in 33 studies including 14 496 patients; 9.4% of patients received a diagnosis of major bleeding (Appendix 1, Figures S22 and S28). Compared with patients treated with systemic thrombolysis, those treated with anticoagulation (OR 0.49, 95% CI 0.36–0.67, I2 = 0%) or CDT (OR 0.61, 95% CI 0.53–0.70, p < 0.0001, I2 = 0%) had lower risks of major bleeding. The certainty of evidence was high for comparisons of anticoagulation with systemic thrombolysis; CDT, however, was associated with low certainty of evidence when compared with systemic thrombolysis (Appendix 1, Figure S28). No statistically significant difference was seen between patients treated with CDT and those treated with anticoagulation (OR 1.24, 95% CI 0.88–1.75, I2 = 0%, low certainty of evidence). Figure 4 shows the comparative risk of major bleeding by treatment.
Network meta-analysis of the association between treatment for pulmonary embolism and major bleeding. Size of squares is proportional to the weight of each arm. Decreased or increased risk of the outcome is of the first type of treatment in comparison, relative to the second type of treatment. The p value indicates the probability of observing the differences between direct and indirect treatment effects. The presence of incoherence is indicated by a p value less than 0.05. Note: AC = anticoagulation, CDT = catheter-directed thrombolysis, CI = confidence interval, OR = odds ratio, ST = systemic thrombolysis.
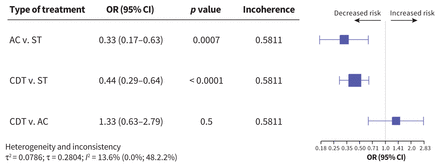
Intracerebral hemorrhage
Intracerebral hemorrhage was specifically assessed and compared in 22 studies, including 20 318 patients (Appendix 1, Figure S23). A total of 1.73% of the patients received a diagnosis of intracerebral hemorrhage. Compared with systemic thrombolysis, CDT (OR 0.44, 95% CI 0.29–0.64, I2 = 13.6%) and anticoagulation (OR 0.33, 95% CI 0.17–0.63, I2 = 13.6%) were associated with decreased risk for intracerebral hemorrhage. The certainty of evidence was moderate for CDT compared with systemic thrombolysis. Comparing anticoagulation with systemic thrombolysis, the level of certainty of evidence was low (Appendix 1, Figure S28).
The risk of ICH was not significantly different between patients treated with anticoagulation and those treated with CDT (OR 1.33, 95% CI 0.63–2.79, I2 = 13.6%, low certainty of evidence). Figure 5 shows the comparative risk of intracerebral hemorrhage by treatment.
Network meta-analysis of the association between treatment for pulmonary embolism and intracranial hemorrhage. Size of squares is proportional to the weight of each arm. Decreased or increased risk of the outcome is of the first type of treatment in comparison, relative to the second type of treatment. The p value indicates the probability of observing the differences between direct and indirect treatment effects. The presence of incoherence is indicated by a p value less than 0.05. Note: AC = anticoagulation, CDT = catheter-directed thrombolysis, CI = confidence interval, OR = odds ratio, ST = systemic thrombolysis.
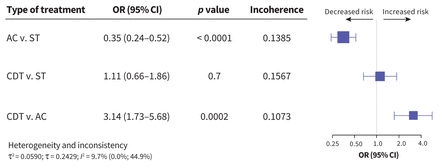
Other bleeding
Minor bleeding was assessed in 20 studies, including 5272 patients (Appendix 1, Figure S24). Patients treated with anticoagulation had a lower risk of minor bleeding than patients treated with systemic thrombolysis (OR 0.35, 95% CI 0.24–0.52, I2 = 9.7%); CDT was also associated with increased risk for minor bleeding compared to anticoagulation (OR 3.14, 95% CI 1.73–5.68, I2 = 9.7%). The risk of minor bleeding was not significantly different between patients receiving CDT and patients receiving systemic thrombolysis (OR 1.11, 95% CI 0.66–1.87, I2 = 9.7%). Figure 6 shows the comparative risk of minor bleeding by treatment.
Network meta-analysis of the association between treatment for pulmonary embolism and minor bleeding. Size of squares is proportional to the weight of each arm. Decreased or increased risk of the outcome is of the first type of treatment in comparison, relative to the second type of treatment. The p value indicates the probability of observing the differences between direct and indirect treatment effects. The presence of incoherence is indicated by a p value less than 0.05. Note: AC = anticoagulation, CDT = catheter-directed thrombolysis, CI = confidence interval, OR = odds ratio, ST = systemic thrombolysis.
The receipt of blood transfusion was assessed in 16 studies, including 12 713 patients (Appendix 1, Figures S2 and S25). Patients treated with CDT had a lower risk of receiving blood transfusion than patients treated with systemic thrombolysis (OR 0.46, 95% CI 0.28–0.77, I2 = 35.1%). The need for blood transfusion was not significantly different between patients treated with CDT and those treated with anticoagulation (OR 0.47, 95% CI 0.21–1.07, I2 = 35.1%).
Gastrointestinal bleeding was assessed in 11 studies, including 15 705 patients (Appendix 1, Figure S3). There were no statistically significant differences between the treatments (Appendix 1, Figure S26).
In terms of death, CDT had the highest P-score. When considering intracerebral hemorrhage, major and minor bleeding, anticoagulation had the highest P-score. When it comes to complications related to bleeding, CDT ranked higher than systemic thrombolysis, except in cases of minor bleeding (Appendix 1, Figure S27).
Subgroup analyses
Among patients with intermediate-to high-risk PE, 6 studies compared CDT to anticoagulation, 7 studies compared systemic thrombolysis to anticoagulation and 2 studies compared CDT to systemic thrombolysis. Network graphs and key characteristics of treatment arms are summarized in Appendix 1, Figure S13 and Table S2. Systemic thrombolysis was associated with a lower risk of death than anticoagulation (OR 2.51, 95% CI 1.03–6.14). No statistically significant difference was observed between patients who received CDT and patients who received systemic therapies (Appendix 1, Figure S12).
Among 19 RCTs, only 1 compared CDT to systemic thrombolysis (Appendix 1, Table S2). No statistically significant differences in risk of death or major bleeding were observed among patients treated with CDT compared with those treated with systemic thrombolysis (Appendix 1, Figures S5 and S6). Among 25 observational studies, 12 compared CDT with systemic thrombolysis. Compared with systemic thrombolysis, CDT was associated with decreased risk of death (OR 0.42, 95% CI 0.31–0.58, I2 = 53.5%), intracerebral hemorrhage (OR 0.46, 95% CI 0.28–0.74, I2 = 32%) and major bleeding (OR 0.61, 95% CI 0.53–0.71, I2 = 1%) (Appendix 1, Figures S5 to S9).
Sensitivity analyses
We conducted sensitivity analyses for primary outcomes, excluding studies that did not have a clear definition of PE severity (defined as “unclear”). Subtracting the “unclear” participants reduced the statistical power of the analysis, especially when comparing CDT with systemic thrombolysis. Results, however, remained unchanged (Appendix 1, Figure S10).
Moreover, we conducted a sensitivity analysis with the studies that were previously excluded because of high risk of bias. In these studies, potential confounders were not properly accounted for (Appendix 1, Table S4). However, the results did not change significantly (Appendix 1, Figure S11).
In addition, we conducted a sensitivity analysis focusing only on in-hospital death; our findings remained similar (Appendix 1, Figure S1).
Publication bias and inconsistency
We found no evidence of publication bias on the funnel plots and by the Egger test (Appendix 1, Figure S14). The risk of bias assessment is summarized in Appendix 1, Figure S28 and Table S3. The Cochran Q statistic showed no general inconsistency in all primary and secondary outcomes (with all p values greater than 0.05). Node-splitting according to a random effect model showed no evidence of incoherence in primary and secondary outcomes analyses (Appendix 1, Figures S15 to S20).
Interpretation
Our network meta-analysis of 44 studies with 20 006 patients showed with moderate certainty that CDT was associated with a lower risk of death than systemic thrombolysis and anticoagulation, a lower risk of intracerebral hemorrhage than systemic thrombolysis and a similar risk of intracerebral hemorrhage as anticoagulation; these findings were based mainly on observational studies. In subgroup analyses of patients with intermediate-high-risk PE, systemic thrombolysis significantly decreased the risk of death compared with anticoagulation. This is of interest, as this subgroup of patients is at serious risk for adverse outcomes and may benefit from more intensive therapy. The large confidence interval in analyses involving patients with intermediate-to high-risk PE treated with CDT suggests that evidence for this subgroup needs further evaluation.
The decreased risk of death among patients treated with CDT may be related to more efficacious treatment of the PE, along with increased safety. Improved efficacy of local treatment (CDT) compared with systemic thrombolysis can be explained by hypoxic pulmonary vasoconstriction, also known as the von Euler–Liljestrand mechanism. According to this mechanism, the consequence of ventilation/perfusion mismatch may divert blood flow from the hypoxic, affected pulmonary circulation to the relatively normal, unaffected circulation, reducing the extent of thrombolysis in the clot area among patients receiving systemic thrombolysis.70,71 In addition, CDT with a multi-hole catheter directly infuses thrombolysis and provides direct contact with the thrombolytic agent to a greater surface area of thrombus.72
As for safety outcomes, we found a statistically significant lower risk for intracerebral hemorrhage, major bleeding and blood transfusion after the acute PE episode among patients treated with CDT compared with patients treated with systemic thrombolysis. Importantly, CDT was not associated with a higher risk of intracerebral hemorrhage and major bleeding compared with anticoagulation. This favourable safety profile of CDT can be explained by the relatively low doses of the thrombolytic agent that are injected locally, reducing the total dose of the lytic drug to 10%–20% compared with systemic thrombolysis, minimizing systemic drug exposure, including within the central nervous system.73–75
In contrast to the lower risk of intracerebral hemorrhage events, the risk of minor bleeding episodes was not lower among patients treated with CDT compared with those treated with systemic thrombolysis. This may be related to the interventional nature of CDT, with postprocedural minor bleeding or hematoma, mainly related to access site, in around 24% of patients.76
Although this study should be interpreted as hypothesis generating, our findings suggest that, among patients eligible for CDT and where facilities exist, CDT should be the preferred treatment, given its effectiveness and the higher toxicity of systemic thrombolysis.
A previous meta-analysis in 2017 assessed the efficacy and safety of catheter-directed interventions in submassive PE.10 Among the 13 studies published, 422 patients received a diagnosis of submassive PE. Pooled mean right ventricle–to–left ventricle ratios and pulmonary artery pressures decreased postintervention. An analysis of safety outcomes showed low pooled rates of in-hospital mortality, major bleeding and minor bleeding. This study, however, had no comparison to a control group.
A systematic review and network meta-analysis published in July 2015 by Jimenez and colleagues9 included 2494 participants from 22 trials. This study compared full-dose and low-dose systemic thrombolysis, CDT (1 study) and anticoagulation. Full-dose thrombolysis, low-dose thrombolysis and CDT trended toward a lower overall mortality rate than anticoagulation, without reaching statistical significance. In terms of risk of death, CDT had the lowest OR at 0.31 (95% CI 0.01–7.96); however, since the CDT arm had a small sample size (59 of 2494 patients), it is not possible to draw any clinical conclusions based on these results.
Limitations
In this analysis, we included RCTs as well as observational studies, some of which involved a small number of patients. However, we excluded observational studies with a substantial risk of bias, according to our strict protocol.
We analyzed all studies involving CDT, with or without fragmentation or ultrasound properties, under 1 arm. In addition, we did not include other endovascular techniques such as suction embolectomy, which makes it impossible to draw any conclusions regarding the preferred method of catheter-directed therapy for PE.
In line with other systematic reviews in the field of CDT, most of the observations and conclusions concerning CDT are based on observational studies.77,78 To minimize selection bias, we excluded observational studies that showed significant baseline differences between groups with respect to PE severity and risk factors. The risk classification of PE and definitions of major bleeding and minor bleeding varied across studies and explicit details regarding these definitions were not always available. Among the 44 included studies, various doses and dose regimes were used. Since the authors did not always provide data on doses and regimens in each study, and different regimes were often used within the same study, we did not describe the specific dose and regimen in each study. The analysis also included various types of thrombolytic and anticoagulation therapies, which may not have been proven to be preferable in terms of efficacy and safety in the management of acute PE.9 Finally, we did not differentiate between low-dose and high-dose systemic thrombolysis since only a very small number of patients received low-dose systemic thrombolysis. Despite these limitations, we believe that our findings should lead to the conduct of appropriate RCTs, such as the ongoing Higher-Risk Pulmonary Embolism Thrombolysis (HI-PEITHO) trial,79 which aims to establish first-line treatment for intermediate-to high-risk PE.
Conclusion
With moderate certainty of evidence, the risk of death and major bleeding, including intracranial hemorrhage, was lower with CDT than with systemic thrombolysis. Compared with anticoagulation, CDT was associated with a lower risk of death and may be associated with similar risk of intracranial hemorrhage and major bleeding, with moderate certainty of evidence. Although these findings were driven mainly by observational data, centres that specialize in CDT can consider it as first-line therapy among patients with intermediate-to high-risk PE.
Acknowledgement
The authors gratefully acknowledge Tomer Ben Shoshan for helping with the search strategy and extracting the records.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work. David Planer, Stav Yanko, Ora Paltiel, Rama Zmiro, Victoria Rotshild and Bruria Hirsh Raccah contributed to the acquisition, analysis and interpretation of data. Stav Yanko, Ora Paltiel, Victoria Rotshild, Offer Amir and Gabby Elbaz-Greener drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. David Planer and Stav Yanko contributed equally to the work as co–first authors.
Funding: No external funding was used for this study.
Data sharing: Data supporting the findings of this study are available from the corresponding author, Bruria Hirsh Raccah, on request.
- Accepted May 16, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/